Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data
- PMID: 24576776
- PMCID: PMC4022989
- DOI: 10.1016/S0140-6736(13)62159-5
Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data
Abstract
Background: Individual participant data meta-analyses of postoperative chemotherapy have shown improved survival for patients with non-small-cell lung cancer (NSCLC). We aimed to do a systematic review and individual participant data meta-analysis to establish the effect of preoperative chemotherapy for patients with resectable NSCLC.
Methods: We systematically searched for trials that started after January, 1965. Updated individual participant data were centrally collected, checked, and analysed. Results from individual randomised controlled trials (both published and unpublished) were combined using a two-stage fixed-effect model. Our primary outcome, overall survival, was defined as the time from randomisation until death (any cause), with living patients censored on the date of last follow-up. Secondary outcomes were recurrence-free survival, time to locoregional and distant recurrence, cause-specific survival, complete and overall resection rates, and postoperative mortality. Prespecified analyses explored any variation in effect by trial and patient characteristics. All analyses were by intention to treat.
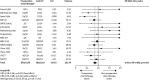
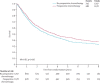
Findings: Analyses of 15 randomised controlled trials (2385 patients) showed a significant benefit of preoperative chemotherapy on survival (hazard ratio [HR] 0·87, 95% CI 0·78-0·96, p=0·007), a 13% reduction in the relative risk of death (no evidence of a difference between trials; p=0·18, I(2)=25%). This finding represents an absolute survival improvement of 5% at 5 years, from 40% to 45%. There was no clear evidence of a difference in the effect on survival by chemotherapy regimen or scheduling, number of drugs, platinum agent used, or whether postoperative radiotherapy was given. There was no clear evidence that particular types of patient defined by age, sex, performance status, histology, or clinical stage benefited more or less from preoperative chemotherapy. Recurrence-free survival (HR 0·85, 95% CI 0·76-0·94, p=0·002) and time to distant recurrence (0·69, 0·58-0·82, p<0·0001) results were both significantly in favour of preoperative chemotherapy although most patients included were stage IB-IIIA. Results for time to locoregional recurrence (0·88, 0·73-1·07, p=0·20), although in favour of preoperative chemotherapy, were not statistically significant.
Interpretation: Findings, which are based on 92% of all patients who were randomised, and mainly stage IB-IIIA, show preoperative chemotherapy significantly improves overall survival, time to distant recurrence, and recurrence-free survival in resectable NSCLC. The findings suggest this is a valid treatment option for most of these patients. Toxic effects could not be assessed.
Funding: Medical Research Council UK.
Copyright © 2014 NSCLC Meta-analysis Collaborative Group. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Treatment for early-stage lung cancer: what next?Lancet. 2014 May 3;383(9928):1528-30. doi: 10.1016/S0140-6736(14)60002-7. Epub 2014 Feb 25. Lancet. 2014. PMID: 24576775 No abstract available.
-
Preoperative chemotherapy for non-small-cell lung cancer.Lancet. 2014 Jul 19;384(9939):232-3. doi: 10.1016/S0140-6736(14)61208-3. Lancet. 2014. PMID: 25042230 No abstract available.
-
Preoperative chemotherapy for non-small-cell lung cancer--authors' reply.Lancet. 2014 Jul 19;384(9939):233. doi: 10.1016/S0140-6736(14)61209-5. Lancet. 2014. PMID: 25042232 No abstract available.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- American Cancer Society . Cancer facts and figures 2007. American Cancer Society; Atlanta: 2007.
-
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123:2096–2103. - PubMed
-
- Berghmans T, Paesmans M, Meert AP. Survival improvement in resectable non-small cell lung cancer with (neo)adjuvant chemotherapy: results of a meta-analysis of the literature. Lung Cancer. 2005;49:13–23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

