Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study
- PMID: 24576907
- PMCID: PMC4896558
- DOI: 10.4161/hv.28022
Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study
Abstract
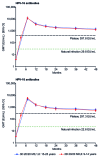
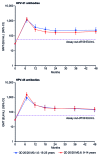
This randomized, partially-blind study (ClinicalTrials.gov registration number NCT00541970) evaluated the immunogenicity and safety of 2-dose (2D) schedules of the HPV-16/18 AS04-adjuvanted vaccine. Results to month (M) 24 have been reported previously and we now report data to M48 focusing on the licensed vaccine formulation (20 μg each of HPV-16 and -18 antigens) administered at M0,6 compared with the standard 3-dose (3D) schedule (M0,1,6). Healthy females (age stratified: 9-14, 15-19, 20-25 years) were randomized to receive 2D at M0,6 (n = 240) or 3D at M0,1,6 (n = 239). In the according-to-protocol immunogenicity cohort, all initially seronegative subjects seroconverted for HPV-16 and -18 antibodies and remained seropositive up to M48. For both HPV-16 and -18, geometric mean antibody titer (GMT) ratios (3D schedule in women aged 15-25 years divided by 2D schedule in girls aged 9-14 years) at M36 and M48 were close to 1, as they were at M7 when non-inferiority was demonstrated. The kinetics of HPV-16, -18, -31, and -45 antibody responses were similar for both groups and HPV-16 and -18 GMTs were substantially higher than natural infection titers. The vaccine had a clinically acceptable safety profile in both groups. In summary, antibody responses to a 2D M0,6 schedule of the licensed vaccine formulation in girls aged 9-14 years appeared comparable to the standard 3D schedule in women aged 15-25 years up to 4 years after first vaccination. A 2D schedule could facilitate implementation of HPV vaccination programs and improve vaccine coverage and series completion rates.
Keywords: administration schedule; female adolescents; human papillomavirus vaccine; immunogenicity; randomized controlled trial; safety; women.
Figures
Similar articles
-
Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study.Hum Vaccin Immunother. 2016;12(1):20-9. doi: 10.1080/21645515.2015.1065363. Epub 2015 Jul 15. Hum Vaccin Immunother. 2016. PMID: 26176261 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study.Hum Vaccin. 2011 Dec;7(12):1374-86. doi: 10.4161/hv.7.12.18322. Epub 2011 Dec 1. Hum Vaccin. 2011. PMID: 22048171 Free PMC article. Clinical Trial.
-
Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9-14 years: Results to month 12 from a randomized trial.Hum Vaccin Immunother. 2015;11(7):1689-702. doi: 10.1080/21645515.2015.1050570. Hum Vaccin Immunother. 2015. PMID: 26062002 Free PMC article. Clinical Trial.
-
AS04-adjuvanted human papillomavirus-16/18 vaccination: recent advances in cervical cancer prevention.Expert Rev Vaccines. 2008 Dec;7(10):1465-73. doi: 10.1586/14760584.7.10.1465. Expert Rev Vaccines. 2008. PMID: 19053203 Review.
-
Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: A systematic review of evidence from clinical trials.Vaccine. 2020 Feb 5;38(6):1302-1314. doi: 10.1016/j.vaccine.2019.12.017. Epub 2019 Dec 20. Vaccine. 2020. PMID: 31870572
Cited by
-
Progress in Vaccination of Prophylactic Human Papillomavirus Vaccine.Front Immunol. 2020 Jul 10;11:1434. doi: 10.3389/fimmu.2020.01434. eCollection 2020. Front Immunol. 2020. PMID: 32754157 Free PMC article. Review.
-
Cancer screening and prevention in low-resource settings.Nat Rev Cancer. 2014 Dec;14(12):822-9. doi: 10.1038/nrc3859. Epub 2014 Oct 30. Nat Rev Cancer. 2014. PMID: 25355377
-
HPV16/18 Antibody Responses After a Single Dose of Nonavalent HPV Vaccine.Pediatrics. 2023 Jul 1;152(1):e2022060301. doi: 10.1542/peds.2022-060301. Pediatrics. 2023. PMID: 37317810 Free PMC article. Clinical Trial.
-
Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study.Hum Vaccin Immunother. 2016;12(1):20-9. doi: 10.1080/21645515.2015.1065363. Epub 2015 Jul 15. Hum Vaccin Immunother. 2016. PMID: 26176261 Free PMC article. Clinical Trial.
-
Immunogenicity of 2 and 3 Doses of the Quadrivalent Human Papillomavirus Vaccine up to 120 Months Postvaccination: Follow-up of a Randomized Clinical Trial.Clin Infect Dis. 2020 Aug 14;71(4):1022-1029. doi: 10.1093/cid/ciz887. Clin Infect Dis. 2020. PMID: 31617568 Free PMC article. Clinical Trial.
References
-
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. , HPV PATRICIA Study Group. . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/10.1016/S0140-6736(09)61248-4; PMID: 19586656 - DOI - PubMed
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al. , HPV PATRICIA Study Group. . Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89 - 99; http://dx.doi.org/10.1016/S1470-2045(11)70286-8; PMID: 22075171 - DOI - PubMed
-
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. , GlaxoSmithKline HPV Vaccine Study Group. . Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 65; http://dx.doi.org/10.1016/S0140-6736(04)17398-4; PMID: 15541448 - DOI - PubMed
-
- Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, et al. , GlaxoSmithKline Vaccine HPV-007 Study Group. . Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 85; http://dx.doi.org/10.1016/S0140-6736(09)61567-1; PMID: 19962185 - DOI - PubMed
-
- Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. . Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.09.049; PMID: 18845199 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
