Necroptosis induced by RIPK3 requires MLKL but not Drp1
- PMID: 24577084
- PMCID: PMC3944236
- DOI: 10.1038/cddis.2014.18
Necroptosis induced by RIPK3 requires MLKL but not Drp1
Abstract
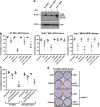
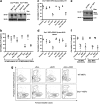
Necroptosis is a mechanism by which cells can kill themselves that does not require caspase activity or the presence of the pro-apoptotic Bcl-2 family members Bax or Bak. It has been reported that RIPK3 (receptor interacting protein kinase 3) activates MLKL (mixed lineage kinase domain-like) to cause cell death that requires dynamin-related protein 1 (Drp1), because survival was increased in cells depleted of Drp1 or treated with the Drp1 inhibitor mdivi-1. To analyze necroptosis in a system that does not require addition of tumor necrosis factor (TNF), we used a construct that allows RIPK3 to be induced in cells, and then dimerized via an E. coli gyrase domain fused to its carboxyl-terminus, using the dimeric gyrase binding antibiotic coumermycin. We have previously shown elsewhere that RIPK3 dimerized in this manner not only induces necroptosis but also apoptosis, which can be inhibited by the broad-spectrum caspase inhibitor Q-VD-OPh (QVD). In response to RIPK3 dimerization, wild-type mouse embryonic fibroblasts (MEFs) underwent cell death that was reduced but not completely blocked by QVD. In contrast, death upon dimerization of RIPK3 in Mlkl(-/-) MEFs was completely inhibited with QVD, confirming that MLKL is required for necroptosis. Similar to wild-type MEFs, most Drp1(-/-) MEFs died when RIPK3 was activated, even in the presence of QVD. Furthermore, overexpression of wild-type MLKL or dominant active mutants of MLKL (Q343A or S345E/S347E) caused death of wild-type and Drp1(-/-) MEFs that was not inhibited with QVD. These results indicate that necroptosis caused by RIPK3 requires MLKL but not Drp1.
Figures

Similar articles
-
Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis.Cell Death Dis. 2014 Jan 16;5(1):e1004. doi: 10.1038/cddis.2013.531. Cell Death Dis. 2014. PMID: 24434512 Free PMC article.
-
TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1.Cell Death Dis. 2013 Jan 17;4(1):e465. doi: 10.1038/cddis.2012.201. Cell Death Dis. 2013. PMID: 23328672 Free PMC article.
-
Interferon-γ induces the cell surface exposure of phosphatidylserine by activating the protein MLKL in the absence of caspase-8 activity.J Biol Chem. 2019 Aug 9;294(32):11994-12006. doi: 10.1074/jbc.RA118.007161. Epub 2019 Jun 19. J Biol Chem. 2019. PMID: 31217278 Free PMC article.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
RIPK3 signaling and its role in the pathogenesis of cancers.Cell Mol Life Sci. 2021 Dec;78(23):7199-7217. doi: 10.1007/s00018-021-03947-y. Epub 2021 Oct 15. Cell Mol Life Sci. 2021. PMID: 34654937 Free PMC article. Review.
Cited by
-
An outline of necrosome triggers.Cell Mol Life Sci. 2016 Jun;73(11-12):2137-52. doi: 10.1007/s00018-016-2189-y. Epub 2016 Apr 6. Cell Mol Life Sci. 2016. PMID: 27052312 Free PMC article. Review.
-
The Killer Pseudokinase Mixed Lineage Kinase Domain-Like Protein (MLKL).Cold Spring Harb Perspect Biol. 2020 Aug 3;12(8):a036376. doi: 10.1101/cshperspect.a036376. Cold Spring Harb Perspect Biol. 2020. PMID: 31712266 Free PMC article. Review.
-
Activation of innate antiviral immune response via double-stranded RNA-dependent RLR receptor-mediated necroptosis.Sci Rep. 2016 Mar 3;6:22550. doi: 10.1038/srep22550. Sci Rep. 2016. PMID: 26935990 Free PMC article.
-
Caspase-8 in inflammatory diseases: a potential therapeutic target.Cell Mol Biol Lett. 2024 Oct 8;29(1):130. doi: 10.1186/s11658-024-00646-x. Cell Mol Biol Lett. 2024. PMID: 39379817 Free PMC article. Review.
-
Necroptosis in Immuno-Oncology and Cancer Immunotherapy.Cells. 2020 Aug 1;9(8):1823. doi: 10.3390/cells9081823. Cells. 2020. PMID: 32752206 Free PMC article. Review.
References
-
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. - PubMed
-
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. - PubMed
-
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. - PubMed
-
- Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell biol. 2000;2:241–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

