Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45γ in human brain malignant glioma (GBM) 8401 cells
- PMID: 24577085
- PMCID: PMC3944240
- DOI: 10.1038/cddis.2014.22
Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45γ in human brain malignant glioma (GBM) 8401 cells
Abstract
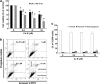
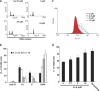
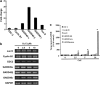
Cucurbitacin E (CuE) is a natural compound previously shown to have anti-feedant, antioxidant and antitumor activities as well as a potent chemo-preventive action against cancer. The present study investigates its anti-proliferative property using MTT assay; CuE demonstrated cytotoxic activity against malignant glioma GBM 8401 cells and induced cell cycle G2/M arrest in these cells. CuE-treated cells accumulated in metaphase (CuE 2.5-10 μM) as determined using MPM-2 by flow cytometry. We attempted to characterize the molecular pathways responsible for cytotoxic effects of CuE in GBM 8401 cells. We studied the genome-wide gene expression profile on microarrays and molecular networks by using pathway analysis tools of bioinformatics. The CuE reduced the expression of 558 genes and elevated the levels of 1354 genes, suggesting an existence of the common pathways involved in induction of G2/M arrest. We identified the RB (GADD45β and GADD45γ) and the p53 (GADD45α) signaling pathways as the common pathways, serving as key molecules that regulate cell cycle. Results indicate that CuE produced G2/M arrest as well as the upregulation of GADD45 γ and binding with CDC2. Both effects increased proportionally with the dose of CuE, suggesting that the CuE-induced mitosis delay is regulated by GADD45γ overexpression. Our findings suggest that, in addition to the known effects on cancer prevention, CuE may have antitumor activity in glioma therapy.
Figures

Similar articles
-
Therapeutic ROS targeting of GADD45γ in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E.Cell Death Dis. 2014 Apr 24;5(4):e1198. doi: 10.1038/cddis.2014.151. Cell Death Dis. 2014. PMID: 24763055 Free PMC article.
-
The effects of cucurbitacin E on GADD45β-trigger G2/M arrest and JNK-independent pathway in brain cancer cells.J Cell Mol Med. 2019 May;23(5):3512-3519. doi: 10.1111/jcmm.14250. Epub 2019 Mar 25. J Cell Mol Med. 2019. PMID: 30912292 Free PMC article.
-
GADD45γ induces G2/M arrest in human pharynx and nasopharyngeal carcinoma cells by cucurbitacin E.Sci Rep. 2014 Sep 23;4:6454. doi: 10.1038/srep06454. Sci Rep. 2014. PMID: 25245461 Free PMC article.
-
Multifaceted Therapeutic Impacts of Cucurbitacin B: Recent Evidences From Preclinical Studies.Phytother Res. 2025 May;39(5):1966-1995. doi: 10.1002/ptr.8454. Epub 2025 Feb 18. Phytother Res. 2025. PMID: 39963741 Review.
-
Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities.Molecules. 2024 Sep 4;29(17):4193. doi: 10.3390/molecules29174193. Molecules. 2024. PMID: 39275042 Free PMC article. Review.
Cited by
-
Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116.Int J Mol Sci. 2018 Aug 2;19(8):2269. doi: 10.3390/ijms19082269. Int J Mol Sci. 2018. PMID: 30072642 Free PMC article.
-
Therapeutic ROS targeting of GADD45γ in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E.Cell Death Dis. 2014 Apr 24;5(4):e1198. doi: 10.1038/cddis.2014.151. Cell Death Dis. 2014. PMID: 24763055 Free PMC article.
-
Cucurbitacin E has neuroprotective properties and autophagic modulating activities on dopaminergic neurons.Oxid Med Cell Longev. 2014;2014:425496. doi: 10.1155/2014/425496. Epub 2014 Dec 9. Oxid Med Cell Longev. 2014. PMID: 25574337 Free PMC article.
-
Roles for GADD45 in Development and Cancer.Adv Exp Med Biol. 2022;1360:23-39. doi: 10.1007/978-3-030-94804-7_2. Adv Exp Med Biol. 2022. PMID: 35505160 Review.
-
Perspectives on natural compounds in chemoprevention and treatment of cancer: an update with new promising compounds.Eur J Cancer. 2021 May;149:165-183. doi: 10.1016/j.ejca.2021.03.009. Epub 2021 Apr 14. Eur J Cancer. 2021. PMID: 33865202 Free PMC article. Review.
References
-
- Lee CC, Wang WH, Lin CF, Chen HH, Chen SC, Lin SC, et al. Malignant transformation of supratentorial ganglioglioma. Clin Neurol Neurosurg. 2012;114:1338–1342. - PubMed
-
- Huang TY, Chang WC, Wang MY, Yang YR, Hsu YC. Effect of sulforaphane on growth inhibition in human brain malignant glioma GBM 8401 cells by means of mitochondrial- and MEK/ERK-mediated apoptosis pathway. Cell Biochem Biophys. 2012;63:247–259. - PubMed
-
- Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, et al. Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs. 2012;23:777–787. - PubMed
-
- Ríos JL, Andújar I, Escandell JM, Giner RM, Recio MC. Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds. Curr Pharm Des. 2012;18:1663–1676. - PubMed
-
- Recio MC, Andujar I, Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem. 2012;19:2088–2103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

