Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy
- PMID: 24577089
- PMCID: PMC3944273
- DOI: 10.1038/cddis.2014.62
Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy
Abstract
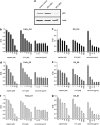
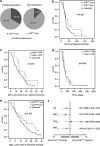
Infection with human papillomaviruses (HPVs) characterizes a distinct subset of head and neck squamous cell cancers (HNSCCs). HPV-positive HNSCC preferentially affect the oropharynx and tonsils. Localized HPV-positive HNSCCs have a favorable prognosis and treatment outcome. However, the impact of HPV in advanced or metastatic HNSCC remains to be defined. In particular, it is unclear whether HPV modulates the response to cetuximab, an antibody targeting the epidermal growth factor receptor (EGFR), which is a mainstay of treatment of advanced HNSCC. To this end, we have examined the sensitivity of HPV-positive and -negative HNSCC models to cetuximab and cytotoxic drugs in vitro and in vivo. In addition, we have stably expressed the HPV oncogenes E6 and E7 in cetuximab-sensitive cancer cell lines to specifically investigate their role in the antibody response. The endogenous HPV status or the expression of HPV oncogenes had no significant impact on cetuximab-mediated suppression of EGFR signaling and proliferation in vitro. Cetuximab effectively inhibited the growth of E6- and E7-expressing tumors grafted in NOD/SCID mice. In support, formalin-fixed, paraffin-embedded tumor samples from cetuximab-treated patients with recurrent or metastatic HNSCC were probed for p16(INK4a) expression, an established biomarker of HPV infection. Response rates (45.5% versus 45.5%) and median progression-free survival (97 versus 92 days) following cetuximab-based therapy were similar in patients with p16(INK4A)-positive and p16(INK4A)-negative tumors. In conclusion, HPV oncogenes do not modulate the anti-EGFR antibody response in HSNCC. Cetuximab treatment should be administered independently of HPV status.
Figures



Similar articles
-
Molecular profile of head and neck squamous cell carcinomas bearing p16 high phenotype.Ann Oncol. 2013 Aug;24(8):2124-31. doi: 10.1093/annonc/mdt013. Epub 2013 Feb 13. Ann Oncol. 2013. PMID: 23406730
-
Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV+ Patients.Clin Cancer Res. 2017 Jun 15;23(12):3072-3083. doi: 10.1158/1078-0432.CCR-16-2203. Epub 2016 Dec 16. Clin Cancer Res. 2017. PMID: 27986750 Free PMC article.
-
Biomarkers of HPV in head and neck squamous cell carcinoma.Cancer Res. 2012 Oct 1;72(19):5004-13. doi: 10.1158/0008-5472.CAN-11-3277. Epub 2012 Sep 18. Cancer Res. 2012. PMID: 22991304 Free PMC article.
-
HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis.Lancet Oncol. 2014 Nov;15(12):1319-31. doi: 10.1016/S1470-2045(14)70471-1. Epub 2014 Oct 16. Lancet Oncol. 2014. PMID: 25439690
-
p16, HPV, and Cetuximab: What Is the Evidence?Oncologist. 2017 Jul;22(7):811-822. doi: 10.1634/theoncologist.2016-0433. Epub 2017 May 18. Oncologist. 2017. PMID: 28526718 Free PMC article. Review.
Cited by
-
Impact of viral presence in tumor on gene expression in non-small cell lung cancer.BMC Cancer. 2018 Aug 22;18(1):843. doi: 10.1186/s12885-018-4748-0. BMC Cancer. 2018. PMID: 30134863 Free PMC article.
-
Long noncoding RNA BC005927 upregulates EPHB4 and promotes gastric cancer metastasis under hypoxia.Cancer Sci. 2018 Apr;109(4):988-1000. doi: 10.1111/cas.13519. Epub 2018 Mar 5. Cancer Sci. 2018. PMID: 29383777 Free PMC article.
-
Increased sensitivity of HPV-positive head and neck cancer cell lines to x-irradiation ± Cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis.Am J Cancer Res. 2015 Feb 15;5(3):1017-31. eCollection 2015. Am J Cancer Res. 2015. PMID: 26045983 Free PMC article.
-
Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies.Clin Cancer Res. 2015 Oct 15;21(20):4597-606. doi: 10.1158/1078-0432.CCR-14-3338. Epub 2015 Jul 2. Clin Cancer Res. 2015. PMID: 26138066 Free PMC article.
-
Targeted Therapy in Oropharyngeal Squamous Cell Carcinoma: The Implications of HPV for Therapy.Rare Cancers Ther. 2015;3(1):89-117. doi: 10.1007/s40487-015-0008-5. Epub 2015 Sep 9. Rare Cancers Ther. 2015. PMID: 27182480 Free PMC article. Review.
References
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. - PubMed
-
- Hong AM, Grulich AE, Jones D, Lee CS, Garland SM, Dobbins TA, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28:3269–3272. - PubMed
-
- Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. - PubMed
-
- Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma. Int J Cancer. 2009;125:362–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

