MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2
- PMID: 24577093
- PMCID: PMC3944261
- DOI: 10.1038/cddis.2014.47
MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2
Abstract
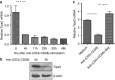
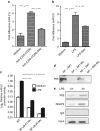
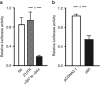
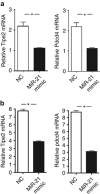
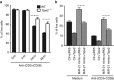
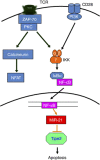
MicroRNAs (MiRs) are short noncoding RNAs that can regulate gene expression. It has been reported that miR-21 suppresses apoptosis in activated T cells, but the molecular mechanism remains undefined. Tumor suppressor Tipe2 (or tumor necrosis factor-α-induced protein 8 (TNFAIP8)-like 2 (TNFAIP8L2)) is a newly identified anti-inflammatory protein of the TNFAIP8 family that is essential for maintaining immune homeostasis. We report here that miR-21 is a direct target of nuclear factor-κB and could regulate Tipe2 expression in a Tipe2 coding region-dependent manner. In activated T cells and macrophages, Tipe2 expression was markedly downregulated, whereas miR-21 expression was upregulated. Importantly, Tipe2-deficient T cells were significantly less sensitive to apoptosis. Conversely, overexpression of Tipe2 in EL-4 T cells increased their susceptibility to activation-induced apoptosis. Therefore, Tipe2 provides a molecular bridge between miR-21 and cell apoptosis; miR-21 suppresses apoptosis in activated T cells at least in part through directly targeting tumor suppressor gene Tipe2.
Figures

References
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
-
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. - PubMed
-
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

