FGF7/KGF regulates autophagy in keratinocytes: A novel dual role in the induction of both assembly and turnover of autophagosomes
- PMID: 24577098
- PMCID: PMC5119059
- DOI: 10.4161/auto.28145
FGF7/KGF regulates autophagy in keratinocytes: A novel dual role in the induction of both assembly and turnover of autophagosomes
Abstract
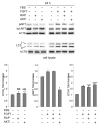
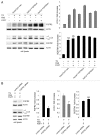
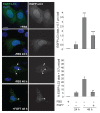
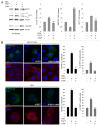
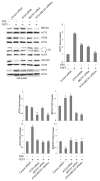
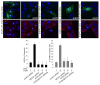
Autophagy is a degradative pathway through which cells overcome stressful conditions and rapidly change their phenotype during differentiation. Despite its protective role, when exacerbated, autophagy may lead to cell death. Several growth factors involved in cell survival and in preventing differentiation are able to inhibit autophagy. Here we investigated the autophagic role of FGF7/KGF, an important player in epithelial cell protection and differentiation. Biochemical and quantitative fluorescence approaches showed that FGF7 and its signaling induce autophagy in human keratinocytes and the use of specific inhibitors indicated that this effect is independent of the PI3K-AKT-MTOR pathway. The selective block of autophagosome-to-lysosome fusion clarified that FGF7 induces autophagy stimulating autophagosome formation. However, quantitative fluorescence approaches also indicated that, upon a prolonged autophagic stimulus, FGF7 is able to accelerate autophagosome turnover. Moreover, in differentiating keratinocytes, the use of the autophagic inhibitor 3-MA as well as the depletion of BECN1 and ATG5, 2 essential regulators of the process, counteracted the FGF7-induced increase of the differentiation marker KRT1/K1, suggesting that autophagy is required for the FGF7-mediated early differentiation. These results provide the first evidence of a role of FGF7 in the regulation of sequential steps of the autophagic process and strengthen the hypothesis of a direct interplay between autophagy and differentiation. On the other hand, the ability of FGF7 to accelerate autophagosome turnover, preventing their dangerous accumulation, is consistent with the well-established protective role played by the growth factor in epithelial cells.
Keywords: FGF7; FGFR2b; KGF; KGFR; autophagy.
Figures





Similar articles
-
The Aberrant Expression of the Mesenchymal Variant of FGFR2 in the Epithelial Context Inhibits Autophagy.Cells. 2019 Jun 29;8(7):653. doi: 10.3390/cells8070653. Cells. 2019. PMID: 31261937 Free PMC article.
-
HPV16 E5 deregulates the autophagic process in human keratinocytes.Oncotarget. 2015 Apr 20;6(11):9370-86. doi: 10.18632/oncotarget.3326. Oncotarget. 2015. PMID: 25826082 Free PMC article.
-
Keratinocyte growth factor promotes melanosome transfer to keratinocytes.J Invest Dermatol. 2005 Dec;125(6):1190-9. doi: 10.1111/j.0022-202X.2005.23929.x. J Invest Dermatol. 2005. PMID: 16354189
-
Evolutionarily conserved role and physiological relevance of a STX17/Syx17 (syntaxin 17)-containing SNARE complex in autophagosome fusion with endosomes and lysosomes.Autophagy. 2013 Oct;9(10):1642-6. doi: 10.4161/auto.25684. Epub 2013 Jul 22. Autophagy. 2013. PMID: 24113031 Review.
-
Coordination of Autophagosome-Lysosome Fusion by Atg8 Family Members.Curr Biol. 2018 Apr 23;28(8):R512-R518. doi: 10.1016/j.cub.2018.02.034. Curr Biol. 2018. PMID: 29689234 Review.
Cited by
-
The Aberrant Expression of the Mesenchymal Variant of FGFR2 in the Epithelial Context Inhibits Autophagy.Cells. 2019 Jun 29;8(7):653. doi: 10.3390/cells8070653. Cells. 2019. PMID: 31261937 Free PMC article.
-
Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment.Bioact Mater. 2024 Mar 1;36:112-125. doi: 10.1016/j.bioactmat.2024.02.023. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38440324 Free PMC article.
-
The Yin and Yang dualistic features of autophagy in thermal burn wound healing.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221125090. doi: 10.1177/03946320221125090. Int J Immunopathol Pharmacol. 2022. PMID: 36121435 Free PMC article.
-
HPV16 E5 deregulates the autophagic process in human keratinocytes.Oncotarget. 2015 Apr 20;6(11):9370-86. doi: 10.18632/oncotarget.3326. Oncotarget. 2015. PMID: 25826082 Free PMC article.
-
5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of Wnt/β-Catenin and Autophagy Pathways and Inhibition of TGF-β Pathways in Dermal Papilla Cells.Molecules. 2022 Mar 28;27(7):2176. doi: 10.3390/molecules27072176. Molecules. 2022. PMID: 35408575 Free PMC article.
References
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. . . Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 2010; 90:1383 - 435; http://dx.doi.org/10.1152/physrev.00030.2009; PMID: 20959619 - DOI - PubMed
-
- Rubinsztein DC, Mariño G, Kroemer G. . Autophagy and aging. Cell 2011; 146:682 - 95; http://dx.doi.org/10.1016/j.cell.2011.07.030; PMID: 21884931 - DOI - PubMed
-
- Cecconi F, Levine B. . The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 2008; 15:344 - 57; http://dx.doi.org/10.1016/j.devcel.2008.08.012; PMID: 18804433 - DOI - PMC - PubMed
-
- Yang Z, Klionsky DJ. . Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22:124 - 31; http://dx.doi.org/10.1016/j.ceb.2009.11.014; PMID: 20034776 - DOI - PMC - PubMed
-
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. . Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8:741 - 52; http://dx.doi.org/10.1038/nrm2239; PMID: 17717517 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
