Role of the basement membrane in regulation of cardiac electrical properties
- PMID: 24577875
- PMCID: PMC4011989
- DOI: 10.1007/s10439-014-0992-x
Role of the basement membrane in regulation of cardiac electrical properties
Abstract
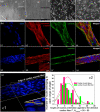
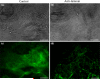
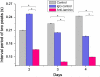
In the heart muscle, each adult cardiomyocyte is enclosed by a basement membrane (BM). This innermost extracellular matrix is a layered assembly of laminin, collagen IV, glycoproteins, and proteoglycans. In this study, the role of the BM network in regulation of the electrical properties of neonatal cardiomyocytes (NCMs) cultured on an aligned collagen I gel was investigated using a multielectrode array (MEA). A laminin antibody was added to the culture medium for 48-120 h to conjugate newly secreted laminin. Then, morphology of the NCMs on an MEA was monitored using a phase contrast microscope, and the BM network that was immunocytostained for laminin was imaged using a fluorescence microscope. When the BM laminin was absent in this culture model, dramatic changes in NCM morphology were observed. Simultaneously, the MEA-recorded cardiac field potential showed changes compared to that from the control groups: The period of contraction shortened to 1/2 of that from the control groups, and the waveform of the calcium influx shifted from a flat plateau to a peak-like waveform, indicating that the electrical properties of the NCMs were closely related to the components and distribution of the BM network.
Figures



References
-
- Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1015–H1026. - PubMed
-
- Borg TK, Gay RE, Johnson LD. Changes in the distribution of fibronectin and collagen during development of the neonatal rat heart. Coll. Relat. Res. 1982;2:211–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

