Myosin IIB and F-actin control apical vacuolar morphology and histamine-induced trafficking of H-K-ATPase-containing tubulovesicles in gastric parietal cells
- PMID: 24578340
- PMCID: PMC3989701
- DOI: 10.1152/ajpgi.00316.2013
Myosin IIB and F-actin control apical vacuolar morphology and histamine-induced trafficking of H-K-ATPase-containing tubulovesicles in gastric parietal cells
Abstract
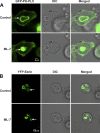
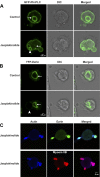
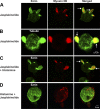
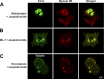
Selective inhibitors of myosin or actin function and confocal microscopy were used to test the role of an actomyosin complex in controlling morphology, trafficking, and fusion of tubulovesicles (TV) containing H-K-ATPase with the apical secretory canaliculus (ASC) of primary-cultured rabbit gastric parietal cells. In resting cells, myosin IIB and IIC, ezrin, and F-actin were associated with ASC, whereas H-K-ATPase localized to intracellular TV. Histamine caused fusion of TV with ASC and subsequent expansion resulting from HCl and water secretion; F-actin and ezrin remained associated with ASC whereas myosin IIB and IIC appeared to dissociate from ASC and relocalize to the cytoplasm. ML-7 (inhibits myosin light chain kinase) caused ASC of resting cells to collapse and most myosin IIB, F-actin, and ezrin to dissociate from ASC. TV were unaffected by ML-7. Jasplakinolide (stabilizes F-actin) caused ASC to develop large blebs to which actin, myosin II, and ezrin, as well as tubulin, were prominently localized. When added prior to stimulation, ML-7 and jasplakinolide prevented normal histamine-stimulated transformations of ASC/TV and the cytoskeleton, but they did not affect cells that had been previously stimulated with histamine. These results indicate that dynamic pools of actomyosin are required for maintenance of ASC structure in resting cells and for trafficking of TV to ASC during histamine stimulation. However, the dynamic pools of actomyosin are not required once the histamine-stimulated transformation of TV/ASC and cytoskeleton has occurred. These results also show that vesicle trafficking in parietal cells shares mechanisms with similar processes in renal collecting duct cells, neuronal synapses, and skeletal muscle.
Keywords: F-actin; membrane dynamics; nonmuscle myosin II; vesicle trafficking.
Figures






Similar articles
-
Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade.J Cell Sci. 1999 Aug;112 ( Pt 16):2639-46. doi: 10.1242/jcs.112.16.2639. J Cell Sci. 1999. PMID: 10413672
-
Myosin II is present in gastric parietal cells and required for lamellipodial dynamics associated with cell activation.Am J Physiol Cell Physiol. 2003 Sep;285(3):C662-73. doi: 10.1152/ajpcell.00085.2003. Epub 2003 Apr 30. Am J Physiol Cell Physiol. 2003. PMID: 12724136
-
Characterization of membrane and cytoskeletal compartments in cultured parietal cells: immunofluorescence and confocal microscopic examination.Eur J Cell Biol. 1993 Feb;60(1):76-87. Eur J Cell Biol. 1993. PMID: 8385019
-
Membrane and protein recycling associated with gastric HCl secretion.J Intern Med Suppl. 1990;732:17-26. doi: 10.1111/j.1365-2796.1990.tb01467.x. J Intern Med Suppl. 1990. PMID: 2166524 Review.
-
Vesicular trafficking machinery, the actin cytoskeleton, and H+-K+-ATPase recycling in the gastric parietal cell.J Physiol. 2001 Apr 15;532(Pt 2):287-96. doi: 10.1111/j.1469-7793.2001.0287f.x. J Physiol. 2001. PMID: 11306650 Free PMC article. Review.
Cited by
-
Gastric Parietal Cell Physiology and Helicobacter pylori-Induced Disease.Gastroenterology. 2019 Jun;156(8):2158-2173. doi: 10.1053/j.gastro.2019.02.036. Epub 2019 Mar 1. Gastroenterology. 2019. PMID: 30831083 Free PMC article. Review.
-
Drosophila motor neuron boutons remodel through membrane blebbing coupled with muscle contraction.Nat Commun. 2023 Jun 8;14(1):3352. doi: 10.1038/s41467-023-38421-9. Nat Commun. 2023. PMID: 37291089 Free PMC article.
-
Regulation of Transporters and Channels by Membrane-Trafficking Complexes in Epithelial Cells.Cold Spring Harb Perspect Biol. 2017 Nov 1;9(11):a027839. doi: 10.1101/cshperspect.a027839. Cold Spring Harb Perspect Biol. 2017. PMID: 28246186 Free PMC article. Review.
References
-
- Agnew BJ, Duman JG, Watson CL, Coling DE, Forte JG. Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J Cell Sci 112: 2639–2646, 1999. - PubMed
-
- Akagi K, Nagao T, Urushidani T. Responsiveness of beta-escin-permeabilized rabbit gastric gland model: effects of functional peptide fragments. Am J Physiol Gastrointest Liver Physiol 277: G736–G744, 1999. - PubMed
-
- Ammar DA, Nguyen PN, Forte JG. Functionally distinct pools of actin in secretory cells. Am J Physiol Cell Physiol 281: C407–C417, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

