The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway
- PMID: 24578385
- PMCID: PMC4047462
- DOI: 10.1074/mcp.M113.034108
The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway
Abstract
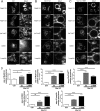
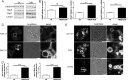
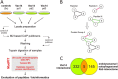
The scaffold protein Vac14 acts in a complex with the lipid kinase PIKfyve and its counteracting phosphatase FIG4, regulating the interconversion of phosphatidylinositol-3-phosphate to phosphatidylinositol-3,5-bisphosphate. Dysfunctional Vac14 mutants, a deficiency of one of the Vac14 complex components, or inhibition of PIKfyve enzymatic activity results in the formation of large vacuoles in cells. How these vacuoles are generated and which processes are involved are only poorly understood. Here we show that ectopic overexpression of wild-type Vac14 as well as of the PIKfyve-binding deficient Vac14 L156R mutant causes vacuoles. Vac14-dependent vacuoles and PIKfyve inhibitor-dependent vacuoles resulted in elevated levels of late endosomal, lysosomal, and autophagy-associated proteins. However, only late endosomal marker proteins were bound to the membranes of these enlarged vacuoles. In order to decipher the linkage between the Vac14 complex and regulators of the endolysosomal pathway, a protein affinity approach combined with multidimensional protein identification technology was conducted, and novel molecular links were unraveled. We found and verified the interaction of Rab9 and the Rab7 GAP TBC1D15 with Vac14. The identified Rab-related interaction partners support the theory that the regulation of vesicular transport processes and phosphatidylinositol-modifying enzymes are tightly interconnected.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Di P. G., De C. P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 - PubMed
-
- Shisheva A. (2012) PIKfyve and its lipid products in health and in sickness. Curr. Top. Microbiol. Immunol. 362, 127–162 - PubMed
-
- Jin N., Chow C. Y., Liu L., Zolov S. N., Bronson R., Davisson M., Petersen J. L., Zhang Y., Park S., Duex J. E., Goldowitz D., Meisler M. H., Weisman L. S. (2008) VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 27, 3221–3234 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

