Cyanobacterial phytochrome2 regulates the heterotrophic metabolism and has a function in the heat and high-light stress response
- PMID: 24578507
- PMCID: PMC3982769
- DOI: 10.1104/pp.113.233270
Cyanobacterial phytochrome2 regulates the heterotrophic metabolism and has a function in the heat and high-light stress response
Abstract
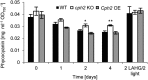
Cyanobacteria combine the photosynthetic and respiratory electron transport in one membrane system, the thylakoid membrane. This feature requires an elaborate regulation mechanism to maintain a certain redox status of the electron transport chain, hence allowing proper photosynthetic and respiratory energy metabolism. In this context, metabolic adaptations, as seen in the light-to-dark and dark-to-light transitions, are particularly challenging. However, the molecular basis of the underlying regulatory mechanisms is not well-understood. Here, we describe a function of cyanobacterial phytochrome2 (Cph2), a phytochrome of the cyanobacterial model system Synechocystis sp. PCC 6803, in regulation of the primary energy metabolism. When cells are shifted from photoautotrophic planktonic growth to light-activated heterotrophic growth and biofilm initiation, knockout of Cph2 results in impaired growth, a decrease in the activity of Glc-6-P dehydrogenase, a decrease of the transcript abundance/activity of cytochrome-c-oxidase, and slower phycocyanin degradation. Measurements of the plastoquinone reduction confirm an impaired heterotrophic metabolism in the cph2 knockout. When cells that were adapted to heterotrophic metabolism are shifted back to light conditions, the knockout of Cph2 results in an altered photosystem II chlorophyll fluorescence induction curve, which is indicative of an impaired redox balance of the electron transport chain. Moreover, Cph2 plays a role in the heat and high-light stress response, particularly under photomixotrophic conditions. Our results show a function of Cph2 in the adaptation of the primary energy metabolism to changing trophic conditions. The physiological role of Cph2 in biofilm formation is discussed.
Figures



Similar articles
-
Thylakoid membrane maturation and PSII activation are linked in greening Synechocystis sp. PCC 6803 cells.Plant Physiol. 2013 Oct;163(2):1037-46. doi: 10.1104/pp.113.224428. Epub 2013 Aug 6. Plant Physiol. 2013. PMID: 23922268 Free PMC article.
-
Inactivation of the petE gene encoding plastocyanin causes different photosynthetic responses in cyanobacterium Synechocystis PCC 6803 under light-dark photoperiod and continuous light conditions.FEMS Microbiol Lett. 2013 Apr;341(2):106-14. doi: 10.1111/1574-6968.12101. Epub 2013 Mar 1. FEMS Microbiol Lett. 2013. PMID: 23397890
-
Distinguishing the Roles of Thylakoid Respiratory Terminal Oxidases in the Cyanobacterium Synechocystis sp. PCC 6803.Plant Physiol. 2016 Jun;171(2):1307-19. doi: 10.1104/pp.16.00479. Epub 2016 Apr 18. Plant Physiol. 2016. PMID: 27208274 Free PMC article.
-
The protein Slr1143 is an active diguanylate cyclase in Synechocystis sp. PCC 6803 and interacts with the photoreceptor Cph2.Microbiology (Reading). 2017 Jun;163(6):920-930. doi: 10.1099/mic.0.000475. Epub 2017 Jun 21. Microbiology (Reading). 2017. PMID: 28635593
-
Regulation of CO2 Concentrating Mechanism in Cyanobacteria.Life (Basel). 2015 Jan 28;5(1):348-71. doi: 10.3390/life5010348. Life (Basel). 2015. PMID: 25636131 Free PMC article. Review.
Cited by
-
Blue and red light significantly aid in the recovery of damaged cells of the cyanobacterium Synechocystis sp. PCC 6803 exposed to ultraviolet radiation.Photochem Photobiol Sci. 2025 Jul;24(7):1137-1152. doi: 10.1007/s43630-025-00749-0. Epub 2025 Jun 13. Photochem Photobiol Sci. 2025. PMID: 40512289
-
Transcriptional regulator PrqR plays a negative role in glucose metabolism and oxidative stress acclimation in Synechocystis sp. PCC 6803.Sci Rep. 2016 Sep 1;6:32507. doi: 10.1038/srep32507. Sci Rep. 2016. PMID: 27582046 Free PMC article.
-
Color Sensing and Signal Transmission Diversity of Cyanobacterial Phytochromes and Cyanobacteriochromes.Mol Cells. 2020 Jun 30;43(6):509-516. doi: 10.14348/molcells.2020.0077. Mol Cells. 2020. PMID: 32438780 Free PMC article. Review.
-
Selective loss of photosystem I and formation of tubular thylakoids in heterotrophically grown red alga Cyanidioschyzon merolae.Photosynth Res. 2019 Jun;140(3):275-287. doi: 10.1007/s11120-018-0603-z. Epub 2018 Nov 10. Photosynth Res. 2019. PMID: 30415289
-
Editorial: Biotechnology of Microalgae, Based on Molecular Biology and Biochemistry of Eukaryotic Algae and Cyanobacteria.Front Microbiol. 2017 Feb 1;8:118. doi: 10.3389/fmicb.2017.00118. eCollection 2017. Front Microbiol. 2017. PMID: 28203229 Free PMC article. No abstract available.
References
-
- Alfonso M, Perewoska I, Constant S, Kirilovsky D. (1999) Redox control of psbA expression in cyanobacteria Synechocystis strains. J Photochem Photobiol B 48: 104–113
-
- Allen MM, Smith AJ. (1969) Nitrogen chlorosis in blue-green algae. Arch Mikrobiol 69: 114–120 - PubMed
-
- Anders K, von Stetten D, Mailliet J, Kiontke S, Sineshchekov VA, Hildebrandt P, Hughes J, Essen LO. (2011) Spectroscopic and photochemical characterization of the red-light sensitive photosensory module of Cph2 from Synechocystis PCC 6803. Photochem Photobiol 87: 160–173 - PubMed
-
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. (1974) Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta 357: 231–245 - PubMed
-
- Asadulghani, Suzuki Y, Nakamoto H. (2003) Light plays a key role in the modulation of heat shock response in the cyanobacterium Synechocystis sp PCC 6803. Biochem Biophys Res Commun 306: 872–879 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

