Solar synthesis: prospects in visible light photocatalysis
- PMID: 24578578
- PMCID: PMC4547527
- DOI: 10.1126/science.1239176
Solar synthesis: prospects in visible light photocatalysis
Abstract
Chemists have long aspired to synthesize molecules the way that plants do-using sunlight to facilitate the construction of complex molecular architectures. Nevertheless, the use of visible light in photochemical synthesis is fundamentally challenging because organic molecules tend not to interact with the wavelengths of visible light that are most strongly emitted in the solar spectrum. Recent research has begun to leverage the ability of visible light-absorbing transition metal complexes to catalyze a broad range of synthetically valuable reactions. In this review, we highlight how an understanding of the mechanisms of photocatalytic activation available to these transition metal complexes, and of the general reactivity patterns of the intermediates accessible via visible light photocatalysis, has accelerated the development of this diverse suite of reactions.
Figures

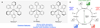



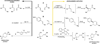
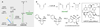
References
-
- Ciamician G. The photochemistry of the future. Science. 1912;36:385–394. - PubMed
-
- Balzani V, Credi A, Venturi M. Photochemical conversion of solar energy. ChemSusChem. 2008;1:26–58. - PubMed
-
- Kalyanasundaram K. Photochemistry of polypyridine and porphyrin complexes. London ; San Diego: Academic Press; 1992. p. 626.
-
- Protti S, Manzini S, Fagnoni M, Albini A. The contribution of photochemistry to green chemistry. Eco-Friendly Synthesis of Fine Chemicals. 2009:80–111.
-
- Esser P, Pohlmann B, Scharf HD. The photochemical-synthesis of fine chemicals with sunlight. Angew. Chem. Int. Ed. 1994;33:2009–2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

