Endothelin-1 promotes cardiomyocyte terminal differentiation in the developing heart via heightened DNA methylation
- PMID: 24578615
- PMCID: PMC3936032
- DOI: 10.7150/ijms.7802
Endothelin-1 promotes cardiomyocyte terminal differentiation in the developing heart via heightened DNA methylation
Abstract
Aims: Hypoxia is a major stress on fetal development and leads to induction of endothelin-1 (ET-1) expression. We tested the hypothesis that ET-1 stimulates the terminal differentiation of cardiomyocytes from mononucleate to binucleate in the developing heart.
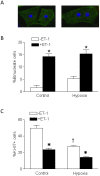
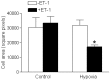
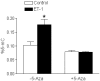
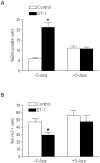
Methods and results: Hypoxia (10.5% O2) treatment of pregnant rats from day 15 to day 21 resulted in a significant increase in prepro-ET-1 mRNA expression in fetal hearts. ET-1 ex vivo treatment of fetal rat cardiomyocytes increased percent binucleate cells and decreased Ki-67 expression, a marker for proliferation, under both control and hypoxic conditions. Hypoxia alone decreased Ki-67 expression and in conjunction with ET-1 treatment decreased cardiomyocyte size. PD145065, a non-selective ET-receptor antagonist, blocked the changes in binucleation and proliferation caused by ET-1. DNA methylation in fetal cardiomyocytes was significantly increased with ET-1 treatment, which was blocked by 5-aza-2'-deoxycytidine, a DNA methylation inhibitor. In addition, 5-aza-2'-deoxycytidine treatment abrogated the increase in binucleation and decrease in proliferation induced by ET-1.
Conclusions: Hypoxic stress and synthesis of ET-1 increases DNA methylation and promotes terminal differentiation of cardiomyocytes in the developing heart. This premature exit of the cell cycle may lead to a reduced cardiomyocyte endowment in the heart and have a negative impact on cardiac function.
Keywords: Endothelin-1; Epigenetic; Fetal development; Heart; Hypoxia.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Newborn hypoxia/anoxia inhibits cardiomyocyte proliferation and decreases cardiomyocyte endowment in the developing heart: role of endothelin-1.PLoS One. 2015 Feb 18;10(2):e0116600. doi: 10.1371/journal.pone.0116600. eCollection 2015. PLoS One. 2015. PMID: 25692855 Free PMC article.
-
Dexamethasone Induces Cardiomyocyte Terminal Differentiation via Epigenetic Repression of Cyclin D2 Gene.J Pharmacol Exp Ther. 2016 Aug;358(2):190-8. doi: 10.1124/jpet.116.234104. Epub 2016 Jun 14. J Pharmacol Exp Ther. 2016. PMID: 27302109 Free PMC article.
-
Dexamethasone Treatment of Newborn Rats Decreases Cardiomyocyte Endowment in the Developing Heart through Epigenetic Modifications.PLoS One. 2015 Apr 29;10(4):e0125033. doi: 10.1371/journal.pone.0125033. eCollection 2015. PLoS One. 2015. PMID: 25923220 Free PMC article.
-
Binucleation of cardiomyocytes: the transition from a proliferative to a terminally differentiated state.Drug Discov Today. 2014 May;19(5):602-9. doi: 10.1016/j.drudis.2013.10.019. Epub 2013 Oct 31. Drug Discov Today. 2014. PMID: 24184431 Free PMC article. Review.
-
Endothelin and pulmonary hypertension.J Cardiovasc Pharmacol. 2000;35(4 Suppl 2):S49-53. doi: 10.1097/00005344-200000002-00012. J Cardiovasc Pharmacol. 2000. PMID: 10976782 Review.
Cited by
-
Near to One's Heart: The Intimate Relationship Between the Placenta and Fetal Heart.Front Physiol. 2018 Jun 26;9:629. doi: 10.3389/fphys.2018.00629. eCollection 2018. Front Physiol. 2018. PMID: 29997513 Free PMC article. Review.
-
Role of DNA methylation in perinatal nicotine-induced development of heart ischemia-sensitive phenotype in rat offspring.Oncotarget. 2017 Aug 10;8(44):76865-76880. doi: 10.18632/oncotarget.20172. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100355 Free PMC article.
-
Gestational Hypoxia and Developmental Plasticity.Physiol Rev. 2018 Jul 1;98(3):1241-1334. doi: 10.1152/physrev.00043.2017. Physiol Rev. 2018. PMID: 29717932 Free PMC article. Review.
-
Maturation of pluripotent stem cell derived cardiomyocytes: The new challenge.Glob Cardiol Sci Pract. 2016 Mar 31;2016(1):e201606. doi: 10.21542/gcsp.2016.6. Glob Cardiol Sci Pract. 2016. PMID: 29043256 Free PMC article. Review.
-
Cardiomyogenesis Modeling Using Pluripotent Stem Cells: The Role of Microenvironmental Signaling.Front Cell Dev Biol. 2019 Aug 9;7:164. doi: 10.3389/fcell.2019.00164. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31448277 Free PMC article. Review.
References
-
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. - PubMed
-
- Clubb FJJr, Bishop SP. Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Laboratory investigation, a journal of technical methods and pathology. 1984;50:571–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

