Removal of the 2-mercaptobenotiazole from model wastewater by ozonation
- PMID: 24578619
- PMCID: PMC3919111
- DOI: 10.1155/2014/173010
Removal of the 2-mercaptobenotiazole from model wastewater by ozonation
Abstract
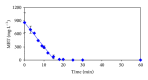
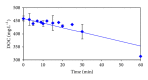
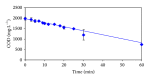
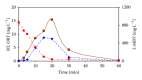
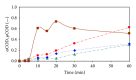
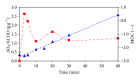
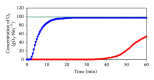
The feasibility of ozonation process for 2-mercaptobenzothiazole (2-MBT) removal follows from results of ozonation of the model wastewater. Total removal of 2-MBT was observed after 20 minutes of ozonation. Very good reproducibility of repeated ozonation trials including sampling and analysis was observed. However, the majority of dissolved organic carbon (DOC) and chemical oxygen demand (COD) remained in the reaction mixture. Benzothiazole (BT) and 2-hydroxybenzothiazole (OBT) intermediates were identified during degradation of 2-MBT with ozone. In addition to the above benzothiazole derivatives, the creation of some other organic compounds follows from results of mass balance. The best fits of experimental data were obtained using the first kinetic model for 2-MBT and zero-order kinetic model for COD and DOC. The reaction time of 60 minutes can be considered as effective with regard to controlled oxidation in order to increase a portion of partially oxidized substances. Higher biodegradability and lower toxicity of ozonation products on respiration activity of activated sludge microorganisms was observed at higher ozonation time.
Figures
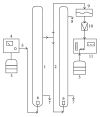
Similar articles
-
Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products.Water Res. 2018 Feb 1;129:486-498. doi: 10.1016/j.watres.2017.10.036. Epub 2017 Oct 20. Water Res. 2018. PMID: 29190578
-
Effect of ozonation on the biodegradability of urban wastewater treatment plant effluent.Sci Total Environ. 2022 Mar 15;812:152466. doi: 10.1016/j.scitotenv.2021.152466. Epub 2021 Dec 21. Sci Total Environ. 2022. PMID: 34952079
-
Modeling ozonation pretreatment parameters of distillery wastewater for improved biodegradability.J Environ Sci Health A Tox Hazard Subst Environ Eng. 2019;54(11):1066-1074. doi: 10.1080/10934529.2019.1631089. Epub 2019 Jun 21. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2019. PMID: 31221003
-
Treatment of soft drink process wastewater by ozonation, ozonation-H₂O₂ and ozonation-coagulation processes.J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47(1):22-30. doi: 10.1080/10934529.2012.629575. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012. PMID: 22217079
-
Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation.Chemosphere. 2015 Feb;121:1-17. doi: 10.1016/j.chemosphere.2014.10.072. Epub 2014 Dec 3. Chemosphere. 2015. PMID: 25479808 Review.
Cited by
-
Removal of Aniline and Benzothiazole Wastewaters Using an Efficient MnO2/GAC Catalyst in a Photocatalytic Fluidised Bed Reactor.Materials (Basel). 2021 Sep 10;14(18):5207. doi: 10.3390/ma14185207. Materials (Basel). 2021. PMID: 34576439 Free PMC article.
-
The Invisible Footprint of Climbing Shoes: High Exposure to Rubber Additives in Indoor Facilities.ACS EST Air. 2025 Apr 24;2(5):930-942. doi: 10.1021/acsestair.5c00017. eCollection 2025 May 9. ACS EST Air. 2025. PMID: 40370931 Free PMC article.
References
-
- Valdés H, Zaror CA, Jekel M. Kinetic study of reactions between ozone and benzothiazole in water. Water Science and Technology. 2003;48(11-12):505–510. - PubMed
-
- de Wewer H, Verachter H. Biodegradation and toxicity of benzothiazoles. Water Research. 1997;31(11):2673–2684.
-
- Valdés H, Zaror CA. Ozonation of benzothiazole saturated-activated carbons: influence of carbon chemical surface properties. Journal of Hazardous Materials. 2006;137(2):1042–1048. - PubMed
-
- Valdés H, Murillo FA, Manoli JA, Zaror CA. Heterogeneous catalytic ozonation of benzothiazole aqueous solution promoted by volcanic sand. Journal of Hazardous Materials. 2008;153(3):1036–1042. - PubMed
-
- De Wever H, Weiss S, Reemtsma T, et al. Comparison of sulfonated and other micropollutants removal in membrane bioreactor and conventional wastewater treatment. Water Research. 2007;41(4):935–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

