Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases
- PMID: 24579667
- PMCID: PMC4806654
- DOI: 10.1615/critreveukaryotgeneexpr.2013006875
Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases
Abstract
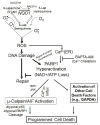
Poly (ADP-ribose) polymerases (PARPs) are a family of related enzymes that share the ability to catalyze the transfer of ADP-ribose to target proteins. PARPs play an important role in various cellular processes, including modulation of chromatin structure, transcription, replication, recombination, and DNA repair. The role of PARP proteins in DNA repair is of particular interest, in view of the finding that certain tumors defective in homologous recombination mechanisms, may rely on PARP-mediated DNA repair for survival, and are sensitive to its inhibition. PARP inhibitors may also increase tumor sensitivity to DNA-damaging agents. Clinical trials of PARP inhibitors are investigating the utility of these approaches in cancer. The hyperactivation of PARP has also been shown to result in a specific programmed cell death pathway involving NAD+/ATP depletion, mu-calpain activation, loss of mitochondrial membrane potential, and the release of apoptosis inducing factor. Hyperactivation of the PARP pathway may be exploited to selectively kill cancer cells. Other PARP forms, including tankyrase 1 (PARP 5a), which plays an important role in enhancing telomere elongation by telomerase, have been found to be potential targets in cancer therapy. The PARP pathway and its inhibition thus offers a number of opportunities for therapeutic intervention in both cancer and other disease states.
Figures
References
-
- Amé J-C, Spenlehauer C, de Murcia G. The PARP super-family. BioEssays. 2004;26(8):882–93. - PubMed
-
- Amé JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Höger T, Ménissier-de Murcia J, de Murcia G. PARP-2, A Novel Mammalian DNA Damage-dependent Poly(ADP-ribose) Polymerase. J Biol Chem. 1999;274(25):17860–8. - PubMed
-
- Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis PJ, Shall S, Zia’ee AA. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979;101(1):135–42. - PubMed
-
- Juarez-Salinas H, Sims JL, Jacobson MK. Poly(ADP-ribose) levels in carcinogen-treated cells. Nature. 1979;282(5740):740–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials