Bright building blocks for chemical biology
- PMID: 24579725
- PMCID: PMC4006396
- DOI: 10.1021/cb500078u
Bright building blocks for chemical biology
Abstract
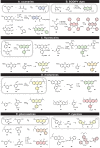
Small-molecule fluorophores manifest the ability of chemistry to solve problems in biology. As we noted in a previous review (Lavis, L. D.; Raines, R. T. ACS Chem. Biol. 2008, 3, 142-155), the extant collection of fluorescent probes is built on a modest set of "core" scaffolds that evolved during a century of academic and industrial research. Here, we survey traditional and modern synthetic routes to small-molecule fluorophores and highlight recent biological insights attained with customized fluorescent probes. Our intent is to inspire the design and creation of new high-precision tools that empower chemical biologists.
Figures

References
-
- de Silva A. P.; Gunaratne H. Q. N.; Gunnlaugsson T.; Huxley A. J. M.; McCoy C. P.; Rademacher J. T.; Rice T. E. (1997) Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566. - PubMed
-
- Johnson I. (1998) Fluorescent probes for living cells. Histochem. J. 30, 123–140. - PubMed
-
- Kiyose K.; Kojima H.; Nagano T. (2008) Functional near-infrared fluorescent probes. Chem. Asian J. 3, 506–515.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

