Phosphatase of regenerating liver: a novel target for cancer therapy
- PMID: 24579927
- PMCID: PMC4216592
- DOI: 10.1517/14728222.2014.892926
Phosphatase of regenerating liver: a novel target for cancer therapy
Abstract
Introduction: Phosphatases of regenerating livers (PRLs) are novel oncogenes that interact with many well-established cell signaling pathways that are misregulated in cancer, and are known to drive cancer metastasis when overexpressed.
Areas covered: This review covers basic information of the discovery and characteristics of the PRL family. We also report findings on the role of PRL in cancer, cell functions and cell signaling. Furthermore, PRL's suitability as a novel drug target is discussed along with current methods being developed to facilitate PRL inhibition.
Expert opinion: PRLs show great potential as novel drug targets for anticancer therapeutics. Studies indicate that PRL can perturb major cancer pathways such as Src/ERK1/2 and PTEN/PI3K/Akt. Upregulation of PRLs has also been shown to drive cancer metastasis. However, in order to fully realize its therapeutic potential, a deeper understanding of the function of PRL in normal tissue and in cancer must be obtained. Novel and integrated biochemical, chemical, biological, and genetic approaches will be needed to identify PRL substrate(s) and to provide proof-of-concept data on the druggability of the PRL phosphatases.
Figures


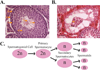
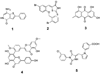
Similar articles
-
Targeting PRL phosphatases in hematological malignancies.Expert Opin Ther Targets. 2024 Apr;28(4):259-271. doi: 10.1080/14728222.2024.2344695. Epub 2024 Apr 26. Expert Opin Ther Targets. 2024. PMID: 38653737 Free PMC article. Review.
-
PRL phosphatases as potential molecular targets in cancer.Mol Cancer Ther. 2005 Nov;4(11):1653-61. doi: 10.1158/1535-7163.MCT-05-0248. Mol Cancer Ther. 2005. PMID: 16275986 Review.
-
Phosphatases of regenerating liver: a novel target in human solid tumors.Chin Med J (Engl). 2008 Aug 5;121(15):1469-74. Chin Med J (Engl). 2008. PMID: 18959128 Review. No abstract available.
-
PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells.Cancer Res. 2007 Jan 15;67(2):643-50. doi: 10.1158/0008-5472.CAN-06-2436. Cancer Res. 2007. PMID: 17234774
-
Molecular mechanisms of the PRL phosphatases.FEBS J. 2013 Jan;280(2):505-24. doi: 10.1111/j.1742-4658.2012.08565.x. Epub 2012 Apr 10. FEBS J. 2013. PMID: 22413991 Review.
Cited by
-
Regulatory Mechanisms and Novel Therapeutic Targeting Strategies for Protein Tyrosine Phosphatases.Chem Rev. 2018 Feb 14;118(3):1069-1091. doi: 10.1021/acs.chemrev.7b00105. Epub 2017 May 25. Chem Rev. 2018. PMID: 28541680 Free PMC article. Review.
-
PRL2 Phosphatase Promotes Oncogenic KIT Signaling in Leukemia Cells through Modulating CBL Phosphorylation.Mol Cancer Res. 2024 Jan 2;22(1):94-103. doi: 10.1158/1541-7786.MCR-23-0115. Mol Cancer Res. 2024. PMID: 37756563 Free PMC article.
-
Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies.Cell Cycle. 2014;13(18):2827-35. doi: 10.4161/15384101.2014.954448. Cell Cycle. 2014. PMID: 25486470 Free PMC article. Review.
-
Targeting Tyrosine Phosphatases: Time to End the Stigma.Trends Pharmacol Sci. 2017 Jun;38(6):524-540. doi: 10.1016/j.tips.2017.03.004. Epub 2017 Apr 12. Trends Pharmacol Sci. 2017. PMID: 28412041 Free PMC article. Review.
-
Targeting PRL phosphatases in hematological malignancies.Expert Opin Ther Targets. 2024 Apr;28(4):259-271. doi: 10.1080/14728222.2024.2344695. Epub 2024 Apr 26. Expert Opin Ther Targets. 2024. PMID: 38653737 Free PMC article. Review.
References
-
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nature Reviews Molecular Cell Biology. 2006;7.11:833–846. - PubMed
-
- Zhang ZY. Protein-tyrosine phosphatases: Biological function, structural characteristics, and mechanism of catalysis. Critical Reviews in Biochemistry and Molecular Biology. 1998;33:1–52. - PubMed
-
-
Mohn KL, Laz TM, Hsu JC, et al. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Molecular and Cellular Biology. 1991;11:381–390. This paper records the discovery of PRL-1 in regenerating liver.
-
-
-
Zeng Q, Hong W, Tan YH. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochemical and Biophysical Research Communications. 1998;244:421–427. PRL 2 and 3 are discovered by Zeng et al.
-
-
- Bessette DC, Qiu D, Pallen CJ. PRL PTPs: Mediators and markers of cancer progression. Cancer Metastasis Reviews. 2008;27(2):231–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
