Ontogeny of adrenal-like glucocorticoid synthesis pathway and of 20α-hydroxysteroid dehydrogenase in the mouse lung
- PMID: 24580729
- PMCID: PMC3944916
- DOI: 10.1186/1756-0500-7-119
Ontogeny of adrenal-like glucocorticoid synthesis pathway and of 20α-hydroxysteroid dehydrogenase in the mouse lung
Abstract
Background: Glucocorticoids exert recognized positive effects on lung development. The genes involved in the classical pathway of glucocorticoid synthesis normally occurring in adrenals were found to be expressed on gestation day (GD) 15.5 in the developing mouse lung. Recently, expression of two of these genes was also detected on GD 17.5 suggesting a more complex temporal regulation than previously expected. Here, we deepen the knowledge on expression of "adrenal" glucocorticoid synthesis genes in the mouse lung during the perinatal period and we also study expression of the gene encoding for the steroid inactivating enzyme 20α-hydroxysteroid dehydrogenase (20α-HSD).
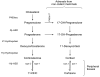
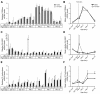
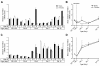
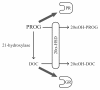
Results: We performed an ontogenic study of P450scc, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase 1 (3β-HSD1), 21-hydroxylase, 11β-hydroxylase, 11β-HSD1, and 11β-HSD2 expression up to post natal day (PN) 15. The substrate (progesterone) and the product (deoxycorticosterone) of 21-hydroxylase are substrates of 20α-HSD, thus 20α-HSD (Akr1c18) gene expression was investigated. In lung samples collected between GD 15.5 and PN 15, 11β-hydroxylase was only detected on GD 15.5. In contrast, all the other tested genes were expressed throughout the analyzed period with different temporal expression patterns. P450scc, 21-hydroxylase, 20α-HSD and 11β-HSD2 mRNA levels increased after birth with different patterns including an increase from PN 3 with a possible sex difference for 21-hydroxylase mRNA. Also, the 21-hydroxylase protein was observed by Western blot in perinatal lungs with higher levels after birth.
Conclusion: Progesterone is present at high levels during gestation and the product of 21-hydroxylase, deoxycorticosterone, can bind the glucocorticoid receptor with an affinity close to that of corticosterone. Detection of 21-hydroxylase at the protein level during antenatal lung development is the first evidence that the adrenal-like glucocorticoid synthesis pathway detected during lung development has the machinery to produce glucocorticoids in the fetal lung. Glucocorticoids from lung 21-hydroxylase appear to modulate lung ontogenesis through paracrine/intracrine actions.
Figures
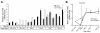

References
-
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

