Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder
- PMID: 24581221
- PMCID: PMC4029264
- DOI: 10.1186/2051-5960-2-25
Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder
Abstract
Background: Aberrant biometal metabolism is a key feature of neurodegenerative disorders including Alzheimer's and Parkinson's diseases. Metal modulating compounds are promising therapeutics for neurodegeneration, but their mechanism of action remains poorly understood. Neuronal ceroid lipofuscinoses (NCLs), caused by mutations in CLN genes, are fatal childhood neurodegenerative lysosomal storage diseases without a cure. We previously showed biometal accumulation in ovine and murine models of the CLN6 variant NCL, but the mechanism is unknown. This study extended the concept that alteration of biometal functions is involved in pathology in these disorders, and investigated molecular mechanisms underlying impaired biometal trafficking in CLN6 disease.
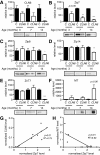
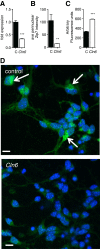
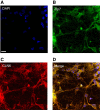
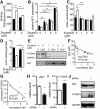
Results: We observed significant region-specific biometal accumulation and deregulation of metal trafficking pathways prior to disease onset in CLN6 affected sheep. Substantial progressive loss of the ER/Golgi-resident Zn transporter, Zip7, which colocalized with the disease-associated protein, CLN6, may contribute to the subcellular deregulation of biometal homeostasis in NCLs. Importantly, the metal-complex, ZnII(atsm), induced Zip7 upregulation, promoted Zn redistribution and restored Zn-dependent functions in primary mouse Cln6 deficient neurons and astrocytes.
Conclusions: This study demonstrates the central role of the metal transporter, Zip7, in the aberrant biometal metabolism of CLN6 variants of NCL and further highlights the key contribution of deregulated biometal trafficking to the pathology of neurodegenerative diseases. Importantly, our results suggest that ZnII(atsm) may be a candidate for therapeutic trials for NCLs.
Figures


Similar articles
-
Altered biometal homeostasis is associated with CLN6 mRNA loss in mouse neuronal ceroid lipofuscinosis.Biol Open. 2013 May 20;2(6):635-46. doi: 10.1242/bio.20134804. Print 2013 Jun 15. Biol Open. 2013. PMID: 23789114 Free PMC article.
-
Deregulation of biometal homeostasis: the missing link for neuronal ceroid lipofuscinoses?Metallomics. 2014 Apr;6(4):932-43. doi: 10.1039/c4mt00032c. Metallomics. 2014. PMID: 24804307
-
X-ray fluorescence imaging reveals subcellular biometal disturbances in a childhood neurodegenerative disorder.Chem Sci. 2014 Jun;5(6):2503-2516. doi: 10.1039/C4SC00316K. Chem Sci. 2014. PMID: 24976945 Free PMC article.
-
Genetics of the neuronal ceroid lipofuscinoses (Batten disease).Biochim Biophys Acta. 2015 Oct;1852(10 Pt B):2237-41. doi: 10.1016/j.bbadis.2015.05.011. Epub 2015 May 27. Biochim Biophys Acta. 2015. PMID: 26026925 Free PMC article. Review.
-
Progress towards understanding disease mechanisms in small vertebrate models of neuronal ceroid lipofuscinosis.Biochim Biophys Acta. 2006 Oct;1762(10):873-89. doi: 10.1016/j.bbadis.2006.08.002. Epub 2006 Aug 10. Biochim Biophys Acta. 2006. PMID: 17023146 Review.
Cited by
-
A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis.Nucleic Acids Res. 2017 Nov 16;45(20):11658-11672. doi: 10.1093/nar/gkx762. Nucleic Acids Res. 2017. PMID: 28977437 Free PMC article.
-
Role of zinc in health and disease.Clin Exp Med. 2024 Feb 17;24(1):38. doi: 10.1007/s10238-024-01302-6. Clin Exp Med. 2024. PMID: 38367035 Free PMC article. Review.
-
Demonstration of subcellular migration of CK2α localization from nucleus to sarco(endo)plasmic reticulum in mammalian cardiomyocytes under hyperglycemia.Mol Cell Biochem. 2018 Jun;443(1-2):25-36. doi: 10.1007/s11010-017-3207-6. Epub 2017 Oct 20. Mol Cell Biochem. 2018. PMID: 29058176
-
Zinc Uptake and Storage During the Formation of the Cerebral Cortex in Mice.Mol Neurobiol. 2019 Oct;56(10):6928-6940. doi: 10.1007/s12035-019-1581-7. Epub 2019 Apr 2. Mol Neurobiol. 2019. PMID: 30941734
-
Restoration of intestinal function in an MPTP model of Parkinson's Disease.Sci Rep. 2016 Jul 29;6:30269. doi: 10.1038/srep30269. Sci Rep. 2016. PMID: 27471168 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

