Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells
- PMID: 24581246
- PMCID: PMC3945717
- DOI: 10.1186/1471-2466-14-32
Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells
Abstract
Background: Cigarette smoke induces a pro-inflammatory response in airway epithelial cells but it is not clear which of the various chemicals contained within cigarette smoke (CS) should be regarded as predominantly responsible for these effects. We hypothesised that acrolein, nicotine and acetylaldehyde, important chemicals contained within volatile cigarette smoke in terms of inducing inflammation and causing addiction, have immunomodulatory effects in primary nasal epithelial cell cultures (PNECs).
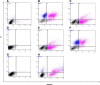
Methods: PNECs from 19 healthy subjects were grown in submerged cultures and were incubated with acrolein, nicotine or acetylaldehyde prior to stimulation with Pseudomonas aeruginosa lipopolysaccharide (PA LPS). Experiments were repeated using cigarette smoke extract (CSE) for comparison. IL-8 was measured by ELISA, activation of NF-κB by ELISA and Western blotting, and caspase-3 activity by Western blotting. Apoptosis was evaluated using Annexin-V staining and the terminal transferase-mediated dUTP nick end-labeling (TUNEL) method.
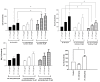
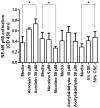
Results: CSE was pro-inflammatory after a 24 h exposure and 42% of cells were apoptotic or necrotic after this exposure time. Acrolein was pro-inflammatory for the PNEC cultures (30 μM exposure for 4 h inducing a 2.0 fold increase in IL-8 release) and also increased IL-8 release after stimulation with PA LPS. In contrast, nicotine had anti-inflammatory properties (0.6 fold IL-8 release after 50 μM exposure to nicotine for 24 h), and acetylaldehyde was without effect. Acrolein and nicotine had cellular stimulatory and anti-inflammatory effects respectively, as determined by NF-κB activation. Both chemicals increased levels of cleaved caspase 3 and induced cell death.
Conclusions: Acrolein is pro-inflammatory and nicotine anti-inflammatory in PNEC cultures. CSE induces cell death predominantly by apoptotic mechanisms.
Figures



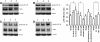
Similar articles
-
Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers.Eur Respir J. 2013 May;41(5):1058-67. doi: 10.1183/09031936.00063112. Epub 2012 Aug 9. Eur Respir J. 2013. PMID: 22878876
-
Cigarette smoke inhibits nasal airway epithelial cell growth and survival.Int Forum Allergy Rhinol. 2013 Mar;3(3):188-92. doi: 10.1002/alr.21129. Epub 2012 Dec 21. Int Forum Allergy Rhinol. 2013. PMID: 23281305
-
Cigarette smoke extract induces cytotoxicity on human nasal epithelial cells.Am J Rhinol. 2007 Mar-Apr;21(2):218-23. doi: 10.2500/ajr.2007.21.2966. Am J Rhinol. 2007. PMID: 17424884
-
The roles and regulatory mechanisms of cigarette smoke constituents in vascular remodeling.Int Immunopharmacol. 2024 Oct 25;140:112784. doi: 10.1016/j.intimp.2024.112784. Epub 2024 Jul 30. Int Immunopharmacol. 2024. PMID: 39083928 Review.
-
Carbonyl Compounds in the Gas Phase of Cigarette Mainstream Smoke and Their Pharmacological Properties.Biol Pharm Bull. 2016;39(6):909-14. doi: 10.1248/bpb.b16-00025. Biol Pharm Bull. 2016. PMID: 27251492 Review.
Cited by
-
Immunological and toxicological risk assessment of e-cigarettes.Eur Respir Rev. 2018 Feb 28;27(147):170119. doi: 10.1183/16000617.0119-2017. Print 2018 Mar 31. Eur Respir Rev. 2018. PMID: 29491036 Free PMC article. Review.
-
Effect of sub-chronic exposure to cigarette smoke, electronic cigarette and waterpipe on human lung epithelial barrier function.BMC Pulm Med. 2020 Aug 12;20(1):216. doi: 10.1186/s12890-020-01255-y. BMC Pulm Med. 2020. PMID: 32787821 Free PMC article.
-
Estimating Dynamic Cellular Morphological Properties via the Combination of the RTCA System and a Hough-Transform-Based Algorithm.Cells. 2019 Oct 21;8(10):1287. doi: 10.3390/cells8101287. Cells. 2019. PMID: 31640200 Free PMC article.
-
The Tobacco Smoke Component, Acrolein, as a Major Culprit in Lung Diseases and Respiratory Cancers: Molecular Mechanisms of Acrolein Cytotoxic Activity.Cells. 2023 Mar 11;12(6):879. doi: 10.3390/cells12060879. Cells. 2023. PMID: 36980220 Free PMC article. Review.
-
Cigarette smoke compounds induce cellular redox imbalance, activate NF-κB, and increase TNF-α/CRP secretion: a possible pathway in the pathogenesis of COPD.Toxicol Res (Camb). 2016 Mar 3;5(3):895-904. doi: 10.1039/c5tx00477b. eCollection 2016 May 1. Toxicol Res (Camb). 2016. PMID: 30090398 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

