Cardiac responses to elevated seawater temperature in Atlantic salmon
- PMID: 24581386
- PMCID: PMC3944800
- DOI: 10.1186/1472-6793-14-2
Cardiac responses to elevated seawater temperature in Atlantic salmon
Abstract
Background: Atlantic salmon aquaculture operations in the Northern hemisphere experience large seasonal fluctuations in seawater temperature. With summer temperatures often peaking around 18-20°C there is growing concern about the effects on fish health and performance. Since the heart has a major role in the physiological plasticity and acclimation to different thermal conditions in fish, we wanted to investigate how three and eight weeks exposure of adult Atlantic salmon to 19°C, previously shown to significantly reduce growth performance, affected expression of relevant genes and proteins in cardiac tissues under experimental conditions.
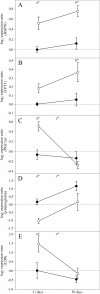
Results: Transcriptional responses in cardiac tissues after three and eight weeks exposure to 19°C (compared to thermal preference, 14°C) were analyzed with cDNA microarrays and validated by expression analysis of selected genes and proteins using real-time qPCR and immunofluorescence microscopy. Up-regulation of heat shock proteins and cell signaling genes may indicate involvement of the unfolded protein response in long-term acclimation to elevated temperature. Increased immunofluorescence staining of inducible nitric oxide synthase in spongy and compact myocardium as well as increased staining of vascular endothelial growth factor in epicardium could reflect induced vascularization and vasodilation, possibly related to increased oxygen demand. Increased staining of collagen I in the compact myocardium of 19°C fish may be indicative of a remodeling of connective tissue with long-term warm acclimation. Finally, higher abundance of transcripts for genes involved in innate cellular immunity and lower abundance of transcripts for humoral immune components implied altered immune competence in response to elevated temperature.
Conclusions: Long-term exposure of Atlantic salmon to 19°C resulted in cardiac gene and protein expression changes indicating that the unfolded protein response, vascularization, remodeling of connective tissue and altered innate immune responses were part of the cardiac acclimation or response to elevated temperature.
Figures



Similar articles
-
Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology.BMC Genomics. 2013 Nov 22;14(1):817. doi: 10.1186/1471-2164-14-817. BMC Genomics. 2013. PMID: 24261939 Free PMC article.
-
Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater.J Fish Biol. 2018 Sep;93(3):550-559. doi: 10.1111/jfb.13683. J Fish Biol. 2018. PMID: 29956316
-
The Innate Immune Response of Atlantic Salmon (Salmo salar) Is Not Negatively Affected by High Temperature and Moderate Hypoxia.Front Immunol. 2020 May 27;11:1009. doi: 10.3389/fimmu.2020.01009. eCollection 2020. Front Immunol. 2020. PMID: 32536921 Free PMC article.
-
Proteomic analysis of temperature-dependent developmental plasticity within the ventricle of juvenile Atlantic salmon (Salmo salar).Curr Res Physiol. 2022 Aug 10;5:344-354. doi: 10.1016/j.crphys.2022.07.005. eCollection 2022. Curr Res Physiol. 2022. PMID: 36035983 Free PMC article.
-
Facing warm temperatures during migration: cardiac mRNA responses of two adult Oncorhynchus nerka populations to warming and swimming challenges.J Fish Biol. 2014 May;84(5):1439-56. doi: 10.1111/jfb.12367. Epub 2014 Mar 31. J Fish Biol. 2014. PMID: 24684400
Cited by
-
The Dynamic Nature of Hypertrophic and Fibrotic Remodeling of the Fish Ventricle.Front Physiol. 2016 Jan 21;6:427. doi: 10.3389/fphys.2015.00427. eCollection 2015. Front Physiol. 2016. PMID: 26834645 Free PMC article.
-
The Atlantic salmon's stress- and immune-related transcriptional responses to moderate hypoxia, an incremental temperature increase, and these challenges combined.G3 (Bethesda). 2021 Jul 14;11(7):jkab102. doi: 10.1093/g3journal/jkab102. G3 (Bethesda). 2021. PMID: 34015123 Free PMC article.
-
Temperature-induced cardiac remodelling in fish.J Exp Biol. 2017 Jan 15;220(Pt 2):147-160. doi: 10.1242/jeb.128496. Epub 2016 Nov 16. J Exp Biol. 2017. PMID: 27852752 Free PMC article. Review.
-
Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart.Antioxidants (Basel). 2021 Aug 31;10(9):1401. doi: 10.3390/antiox10091401. Antioxidants (Basel). 2021. PMID: 34573033 Free PMC article. Review.
-
Vascular compliance and left ventricular compliance cross talk: Implications for using long-term heat acclimation in cardiac care.Front Physiol. 2023 Mar 7;14:1074391. doi: 10.3389/fphys.2023.1074391. eCollection 2023. Front Physiol. 2023. PMID: 36960151 Free PMC article. Review.
References
-
- Brett JR. Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka) Am Zool. 1971;11:99–113.
-
- Fry FEJ. In: Fish Physiology. Hoar WD, Randall DJ, editor. New York: Academic; 1971. The effect of environmental factors on the physiology of fish.
-
- Handeland SO, Bjornsson BT, Arnesen AM, Stefansson SO. Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture. 2003;220(1–4):367–384.
-
- Claireaux G, Webber DM, Lagardere JP, Kerr SR. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua) J Sea Res. 2000;44(3–4):257–265.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

