Outcome markers for clinical trials in cerebral amyloid angiopathy
- PMID: 24581702
- PMCID: PMC4085787
- DOI: 10.1016/S1474-4422(14)70003-1
Outcome markers for clinical trials in cerebral amyloid angiopathy
Abstract
Efforts are underway for early-phase trials of candidate treatments for cerebral amyloid angiopathy, an untreatable cause of haemorrhagic stroke and vascular cognitive impairment. A major barrier to these trials is the absence of consensus on measurement of treatment effectiveness. A range of potential outcome markers for cerebral amyloid angiopathy can be measured against the ideal criteria of being clinically meaningful, closely representative of biological progression, efficient for small or short trials, reliably measurable, and cost effective. In practice, outcomes tend either to have high clinical salience but low statistical efficiency, and thus more applicability for late-phase studies, or greater statistical efficiency but more limited clinical meaning. The most statistically efficient markers might be those that are potentially reversible with treatment, although their clinical significance remains unproven. Many of the candidate outcomes for cerebral amyloid angiopathy trials are probably applicable also to other small-vessel brain diseases.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
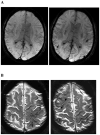


Comment in
-
Developing biomarkers for cerebral amyloid angiopathy trials: do potential disease phenotypes hold promise?Lancet Neurol. 2014 Jun;13(6):538-40. doi: 10.1016/S1474-4422(14)70096-1. Lancet Neurol. 2014. PMID: 24849855 No abstract available.
-
Developing biomarkers for cerebral amyloid angiopathy trials: do potential disease phenotypes hold promise? - authors' reply.Lancet Neurol. 2014 Jun;13(6):540. doi: 10.1016/S1474-4422(14)70097-3. Lancet Neurol. 2014. PMID: 24849857 Free PMC article. No abstract available.
References
-
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston Criteria. Neurology. 2001;56(4):537–9. - PubMed
-
- Pfizer. Study evaluating the safety, tolerability and efficacy of PF-04360365 in adults with probable cerebral amyloid angiopathy. ClinicalTrialsgov. 2013:NCT01821118. Available from: http://clinicaltrials.gov/ct2/show/NCT01821118?term=ponezumab&rank=5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

