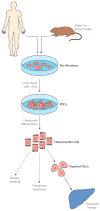
Cellular therapy for liver disease
- PMID: 24582199
- PMCID: PMC4212517
- DOI: 10.1016/j.mayocp.2013.10.023
Cellular therapy for liver disease
Abstract
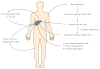
Regenerative medicine is energizing and empowering basic science and has the potential to dramatically transform health care in the future. Given the remarkable intrinsic regenerative properties of the liver, as well as widespread adoption of regenerative strategies for liver disease (eg, liver transplant, partial hepatectomy, living donor transplant), hepatology has always been at the forefront of clinical regenerative medicine. However, an expanding pool of patients awaiting liver transplant, a limited pool of donor organs, and finite applicability of the current surgical approaches have created a need for more refined and widely available regenerative medicine strategies. Although cell-based therapies have been used extensively for hematologic malignant diseases and other conditions, the potential application of cellular therapy for acute and chronic liver diseases has only more recently been explored. New understanding of the mechanisms of liver regeneration and repair, including activation of local stem/progenitor cells and contributions from circulating bone marrow-derived stem cells, provide the theoretical underpinnings for the rational use of cell-based therapies in clinical trials. In this review, we dissect the scientific rationale for various modalities of cell therapy for liver diseases being explored in animal models and review those tested in human clinical trials. We also attempt to clarify some of the important ongoing questions that need to be addressed in order to bring these powerful therapies to clinical translation. Discussions will cover transplant of hepatocytes and liver stem/progenitor cells as well as infusion or stimulation of bone marrow-derived stem cells. We also highlight tremendous scientific advances on the horizon, including the potential use of induced pluripotent stem cells and their derivatives as individualized regenerative therapy for liver disease.
Copyright © 2014 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Dienstag JL, Cosimi AB. Liver transplantation—a vision realized. N Engl J Med. 2012;367(16):1483–1485. - PubMed
-
- Terzic A, Nelson TJ. Regenerative medicine primer. Mayo Clin Proc. 2013;88(7):766–775. - PubMed
-
- Matteson EL, Terzic A. Introduction to the Symposium on Regenerative Medicine. Mayo Clin Proc. 2013;88(7):645–646. - PubMed
-
- McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis. 2008;28(2):210–217. - PubMed
-
- Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7(5):288–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources