Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial
- PMID: 24582471
- PMCID: PMC4176708
- DOI: 10.1016/S0140-6736(14)60222-1
Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial
Abstract
Background: Amyotrophic lateral sclerosis is a fatal neurodegenerative disease with few therapeutic options. Mild obesity is associated with greater survival in patients with the disease, and calorie-dense diets increased survival in a mouse model. We aimed to assess the safety and tolerability of two hypercaloric diets in patients with amyotrophic lateral sclerosis receiving enteral nutrition.
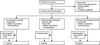
Methods: In this double-blind, placebo-controlled, randomised phase 2 clinical trial, we enrolled adults with amyotrophic lateral sclerosis from participating centres in the USA. Eligible participants were aged 18 years or older with no history of diabetes or liver or cardiovascular disease, and who were already receiving percutaneous enteral nutrition. We randomly assigned participants (1:1:1) using a computer-generated list of random numbers to one of three dietary interventions: replacement calories using an isocaloric tube-fed diet (control), a high-carbohydrate hypercaloric tube-fed diet (HC/HC), or a high-fat hypercaloric tube-fed diet (HF/HC). Participants received the intervention diets for 4 months and were followed up for 5 months. The primary outcomes were safety and tolerability, analysed in all patients who began their study diet. This trial is registered with ClinicalTrials.gov, number NCT00983983.
Findings: Between Dec 14, 2009, and Nov 2, 2012, we enrolled 24 participants, of whom 20 started their study diet (six in the control group, eight in the HC/HC group, and six in the HF/HC group). One patient in the control group, one in the HC/HC group, and two in the HF/HC group withdrew consent before receiving the intervention. Participants who received the HC/HC diet had a smaller total number of adverse events than did those in the other groups (23 in the HC/HC group vs 42 in the control group vs 48 in the HF/HC group; overall, p=0.06; HC/HC vs control, p=0.06) and significantly fewer serious adverse events than did those on the control diet (none vs nine; p=0.0005). Fewer patients in the HC/HC group discontinued their study diet due to adverse events (none [0%] of eight in the HC/HC group vs three [50%] of six in the control group). During the 5 month follow-up, no deaths occurred in the nine patients assigned to the HC/HC diet compared with three deaths (43%) in the seven patients assigned to the control diet (log-rank p=0.03). Adverse events, tolerability, deaths, and disease progression did not differ significantly between the HF/HC group and the control group.
Interpretation: Our results provide preliminary evidence that hypercaloric enteral nutrition is safe and tolerable in patients with amyotrophic lateral sclerosis, and support the study of nutritional interventions in larger randomised controlled trials at earlier stages of the disease.
Funding: Muscular Dystrophy Association, National Center for Research Resources, National Institutes of Health, and Harvard NeuroDiscovery Center.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Anne-Marie Wills M.D., M.P.H. has received research funding from the Muscular Dystrophy Association, NIH/NINDS, Schering-Plough and consultant payments from Asubio pharmaceuticals and Accordant, a CVS/Caremark disease management company.
Jane Hubbard M.S., R.D. reports no conflicts of interest/financial disclosures.
Eric A. Macklin Ph.D. serves on DSMB's for Lantheus Medical Imaging and Shire Human Genetic Therapies and was an unpaid consultant to Knopp Biosciences.
Jonathan Glass, M.D. receives research funding from MDA, ALSA, NIH, and Neuralstem, Inc.
Rup Tandan, M.D. F.R.C.P. has received research funding from the Muscular Dystrophy Association, NIH/NINDS, National ALS Association, Novartis Pharmaceuticals and Alexion Pharmaceuticals; speakers bureau payments from Athena Diagnostics; and consultant payments from Rx Solutions and Walgreens.
Ericka P Simpson, M.D. reports no conflicts of interest/financial disclosures.
Benjamin Brooks, M.D. reports grants from Muscular Dystrophy Association; grants from Cytokinetics Pharmaceuticals, Biogen-Idec Pharmaceuticals, Avanir Pharmaceuticals, and from Carolinas ALS Research Fund Carolinas Healthcare Foundation, He has served as a consultant for Cytokinetics Pharmaceuticals, Knopp Biosciences, the American Academy of Neurology, Asubio Pharmaceuticals, Bristol-Myers- Squibb Pharmaceuticals, Countervail Corporation, Biogen-Idec Pharmaceuticals, and is an unpaid member of the Board of Directors of the ALS Research Group.
Deborah Gelinas, M.D. serves on the Speaker Bureau for Avanir Pharmaceuticals.
Hiroshi Mitsumoto, M.D. reports no conflicts of interest/financial disclosures.
Tahseen Mozaffar, M.D. reports no conflicts of interest/financial disclosures.
Gregory P. Hanes M.D. reports no conflicts of interest/financial disclosures.
Shafeeq S. Ladha M.D. reports no conflicts of interest/financial disclosures.
Terry Heiman-Patterson, M.D. reports no conflicts of interest/financial disclosures.
Jonathan Katz, M.D. reports no conflicts of interest/financial disclosures.
Jau-Shin Lou, M.D. Ph.D reports no conflicts of interest/financial disclosures.
Katy Mahoney B.A. reports no conflicts of interest/financial disclosures.
Daniela Grasso B.A. reports no conflicts of interest/financial disclosures.
Robert Lawson B.S. reports no conflicts of interest/financial disclosures.
Hong Yu M.S. reports no conflicts of interest/financial disclosures.
Merit Cudkowicz, M.D. M.Sc. reports no conflicts of interest/financial disclosures.
Figures

Comment in
-
High-calorie diets in amyotrophic lateral sclerosis.Lancet. 2014 Jun 14;383(9934):2028-2030. doi: 10.1016/S0140-6736(14)60270-1. Epub 2014 Feb 28. Lancet. 2014. PMID: 24582470 No abstract available.
-
Motor neuron disease: High-calorie diet might delay amyotrophic lateral sclerosis.Nat Rev Neurol. 2014 Apr;10(4):181. doi: 10.1038/nrneurol.2014.40. Epub 2014 Mar 11. Nat Rev Neurol. 2014. PMID: 24614518 No abstract available.
References
-
- Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. - PubMed
-
- Kasarskis EJ, Neville HE. Management of ALS: nutritional care. Neurology. 1996;47(4) Suppl 2:S118–S120. - PubMed
-
- Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10(1):75–82. - PubMed
-
- Genton L, Viatte V, Janssens JP, Heritier AC, Pichard C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin Nutr. 2011;30(5):553–559. - PubMed
-
- Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence–based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1218–1226. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

