Comparative bioinformatics, temporal and spatial expression analyses of Ixodes scapularis organic anion transporting polypeptides
- PMID: 24582512
- PMCID: PMC4432481
- DOI: 10.1016/j.ttbdis.2013.12.002
Comparative bioinformatics, temporal and spatial expression analyses of Ixodes scapularis organic anion transporting polypeptides
Abstract
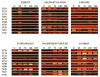
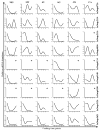
Organic anion-transporting polypeptides (Oatps) are an integral part of the detoxification mechanism in vertebrates and invertebrates. These cell surface proteins are involved in mediating the sodium-independent uptake and/or distribution of a broad array of organic amphipathic compounds and xenobiotic drugs. This study describes bioinformatics and biological characterization of 9 Oatp sequences in the Ixodes scapularis genome. These sequences have been annotated on the basis of 12 transmembrane domains, consensus motif D-X-RW-(I,V)-GAWW-X-G-(F,L)-L, and 11 conserved cysteine amino acid residues in the large extracellular loop 5 that characterize the Oatp superfamily. Ixodes scapularis Oatps may regulate non-redundant cross-tick species conserved functions in that they did not cluster as a monolithic group on the phylogeny tree and that they have orthologs in other ticks. Phylogeny clustering patterns also suggest that some tick Oatp sequences transport substrates that are similar to those of body louse, mosquito, eye worm, and filarial worm Oatps. Semi-quantitative RT-PCR analysis demonstrated that all 9 I. scapularis Oatp sequences were expressed during tick feeding. Ixodes scapularis Oatp genes potentially regulate functions during early and/or late-stage tick feeding as revealed by normalized mRNA profiles. Normalized transcript abundance indicates that I. scapularis Oatp genes are strongly expressed in unfed ticks during the first 24h of feeding and/or at the end of the tick feeding process. Except for 2 I. scapularis Oatps, which were expressed in the salivary glands and ovaries, all other genes were expressed in all tested organs, suggesting the significance of I. scapularis Oatps in maintaining tick homeostasis. Different I. scapularis Oatp mRNA expression patterns were detected and discussed with reference to different physiological states of unfed and feeding ticks.
Keywords: Expression analyses; Ixodes scapularis; Organic anion transporting polypeptides.
Copyright © 2014 Elsevier GmbH. All rights reserved.
Figures







Similar articles
-
Molecular and biological characterization of the Amblyomma americanum organic anion transporter polypeptide.J Exp Biol. 2008 Nov;211(Pt 21):3401-8. doi: 10.1242/jeb.022376. J Exp Biol. 2008. PMID: 18931313
-
Characterization of tick organic anion transporting polypeptides (OATPs) upon bacterial and viral infections.Parasit Vectors. 2018 Nov 14;11(1):593. doi: 10.1186/s13071-018-3160-6. Parasit Vectors. 2018. PMID: 30428915 Free PMC article.
-
Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties.Pflugers Arch. 2004 Feb;447(5):653-65. doi: 10.1007/s00424-003-1168-y. Epub 2003 Oct 25. Pflugers Arch. 2004. PMID: 14579113 Review.
-
Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis.BMC Genomics. 2009 May 12;10:217. doi: 10.1186/1471-2164-10-217. BMC Genomics. 2009. PMID: 19435496 Free PMC article.
-
Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family.Xenobiotica. 2008 Jul;38(7-8):778-801. doi: 10.1080/00498250801986951. Xenobiotica. 2008. PMID: 18668430 Review.
Cited by
-
Human rickettsial pathogen modulates arthropod organic anion transporting polypeptide and tryptophan pathway for its survival in ticks.Sci Rep. 2017 Oct 16;7(1):13256. doi: 10.1038/s41598-017-13559-x. Sci Rep. 2017. PMID: 29038575 Free PMC article.
-
Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step.Polymers (Basel). 2022 Jul 31;14(15):3124. doi: 10.3390/polym14153124. Polymers (Basel). 2022. PMID: 35956639 Free PMC article.
-
First draft genome for the sand-hopper Trinorchestia longiramus.Sci Data. 2020 Mar 9;7(1):85. doi: 10.1038/s41597-020-0424-8. Sci Data. 2020. PMID: 32152293 Free PMC article.
-
Tick Saliva and Salivary Glands: What Do We Know So Far on Their Role in Arthropod Blood Feeding and Pathogen Transmission.Front Cell Infect Microbiol. 2022 Jan 19;11:816547. doi: 10.3389/fcimb.2021.816547. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35127563 Free PMC article. Review.
-
Effect of Chemical Surface Texturing on the Superhydrophobic Behavior of Micro-Nano-Roughened AA6082 Surfaces.Materials (Basel). 2021 Nov 24;14(23):7161. doi: 10.3390/ma14237161. Materials (Basel). 2021. PMID: 34885310 Free PMC article.
References
-
- Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. - PubMed
-
- Abe T, Kakyo M, Tokui T, Nagakomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. - PubMed
-
- Alekseev AN, Semenov AV, Dubinina HV. Evidence of Babesia microti infection in multi-infected Ixodes persulcatus ticks in Russia. Exp Appl Acarol. 2003;29:345–353. - PubMed
-
- Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–860. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

