Despite normal arteriogenic and angiogenic responses, hind limb perfusion recovery and necrotic and fibroadipose tissue clearance are impaired in matrix metalloproteinase 9-deficient mice
- PMID: 24582703
- PMCID: PMC4147032
- DOI: 10.1016/j.jvs.2014.01.038
Despite normal arteriogenic and angiogenic responses, hind limb perfusion recovery and necrotic and fibroadipose tissue clearance are impaired in matrix metalloproteinase 9-deficient mice
Abstract
Objective: The relative contributions of arteriogenesis, angiogenesis, and ischemic muscle tissue composition toward reperfusion after arterial occlusion are largely unknown. Differential loss of bone marrow-derived cell (BMC) matrix metalloproteinase 9 (MMP9), which has been implicated in all of these processes, was used to assess the relative contributions of these processes during limb reperfusion.
Methods: We compared collateral growth (arteriogenesis), capillary growth (angiogenesis), and ischemic muscle tissue composition after femoral artery ligation in FVB/NJ mice that had been reconstituted with bone marrow from wild-type or MMP9(-/-) mice.
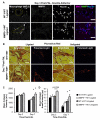
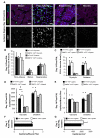
Results: Laser Doppler perfusion imaging confirmed decreased reperfusion capacity in mice with BMC-specific loss of MMP9; however, collateral arteriogenesis was not affected. Furthermore, when accounting for the fact that muscle tissue composition changes markedly with ischemia (ie, necrotic, fibroadipose, and regenerating tissue regions are present), angiogenesis was also unaffected. Instead, BMC-specific loss of MMP9 caused an increase in the proportion of necrotic and fibroadipose tissue, which showed the strongest correlation with poor perfusion recovery. Similarly, the reciprocal loss of MMP9 from non-BMCs showed similar deficits in perfusion and tissue composition without affecting arteriogenesis.
Conclusions: By concurrently analyzing arteriogenesis, angiogenesis, and ischemic tissue composition, we determined that the loss of BMC-derived or non-BMC-derived MMP9 impairs necrotic and fibroadipose tissue clearance after femoral artery ligation, despite normal arteriogenic and angiogenic vascular growth. These findings imply that therapeutic revascularization strategies for treating peripheral arterial disease may benefit from additionally targeting necrotic tissue clearance or skeletal muscle regeneration, or both.
Copyright © 2015 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
P2Y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia.J Vasc Surg. 2016 Jan;63(1):216-25. doi: 10.1016/j.jvs.2014.06.112. Epub 2014 Jul 31. J Vasc Surg. 2016. PMID: 25088742 Free PMC article.
-
Tumor suppressor protein p53 negatively regulates ischemia-induced angiogenesis and arteriogenesis.J Vasc Surg. 2018 Dec;68(6S):222S-233S.e1. doi: 10.1016/j.jvs.2018.02.055. Epub 2018 Aug 17. J Vasc Surg. 2018. PMID: 30126780 Free PMC article.
-
Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis.J Vasc Surg. 2009 Feb;49(2):464-73. doi: 10.1016/j.jvs.2008.08.077. Epub 2008 Nov 22. J Vasc Surg. 2009. PMID: 19028053 Free PMC article.
-
Systematic Interrogation of Angiogenesis in the Ischemic Mouse Hind Limb: Vulnerabilities and Quality Assurance.Arterioscler Thromb Vasc Biol. 2020 Oct;40(10):2454-2467. doi: 10.1161/ATVBAHA.120.315028. Epub 2020 Aug 13. Arterioscler Thromb Vasc Biol. 2020. PMID: 32787524 Free PMC article.
-
Epigenetic regulators of the revascularization response to chronic arterial occlusion.Cardiovasc Res. 2019 Mar 15;115(4):701-712. doi: 10.1093/cvr/cvz001. Cardiovasc Res. 2019. PMID: 30629133 Free PMC article. Review.
Cited by
-
Computational Network Model Prediction of Hemodynamic Alterations Due to Arteriolar Rarefaction and Estimation of Skeletal Muscle Perfusion in Peripheral Arterial Disease.Microcirculation. 2015 Jul;22(5):360-9. doi: 10.1111/micc.12203. Microcirculation. 2015. PMID: 25866235 Free PMC article.
-
Factor VII activating protease (FSAP) influences vascular remodeling in the mouse hind limb ischemia model.Am J Transl Res. 2017 Jun 15;9(6):3084-3095. eCollection 2017. Am J Transl Res. 2017. PMID: 28670395 Free PMC article.
-
DNA Methyltransferase 1-Dependent DNA Hypermethylation Constrains Arteriogenesis by Augmenting Shear Stress Set Point.J Am Heart Assoc. 2017 Nov 30;6(12):e007673. doi: 10.1161/JAHA.117.007673. J Am Heart Assoc. 2017. PMID: 29191807 Free PMC article.
-
The Role of Circulating Biomarkers in Peripheral Arterial Disease.Int J Mol Sci. 2021 Mar 30;22(7):3601. doi: 10.3390/ijms22073601. Int J Mol Sci. 2021. PMID: 33808453 Free PMC article. Review.
-
The partitioning of nanoparticles to endothelium or interstitium during ultrasound-microbubble-targeted delivery depends on peak-negative pressure.J Nanopart Res. 2015 Aug;17(8):345. doi: 10.1007/s11051-015-3153-8. Epub 2015 Aug 22. J Nanopart Res. 2015. PMID: 26594129 Free PMC article.
References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J.Vasc.Surg. :S5–67. - PubMed
-
- Van Weel V, van Tongeren RB, van Hinsbergh V, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann.Vasc.Surg. :582–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

