Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease
- PMID: 24582968
- PMCID: PMC4005604
- DOI: 10.1016/j.pharmthera.2014.02.007
Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease
Abstract
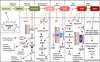
The cholesterol biosynthesis pathway, also known as the mevalonate (MVA) pathway, is an essential cellular pathway that is involved in diverse cell functions. The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (HMGCR) is the rate-limiting step in cholesterol biosynthesis and catalyzes the conversion of HMG-CoA to MVA. Given its role in cholesterol and isoprenoid biosynthesis, the regulation of HMGCR has been intensely investigated. Because all cells require a steady supply of MVA, both the sterol (i.e. cholesterol) and non-sterol (i.e. isoprenoid) products of MVA metabolism exert coordinated feedback regulation on HMGCR through different mechanisms. The proper functioning of HMGCR as the proximal enzyme in the MVA pathway is essential under both normal physiologic conditions and in many diseases given its role in cell cycle pathways and cell proliferation, cholesterol biosynthesis and metabolism, cell cytoskeletal dynamics and stability, cell membrane structure and fluidity, mitochondrial function, proliferation, and cell fate. The blockbuster statin drugs ('statins') directly bind to and inhibit HMGCR, and their use for the past thirty years has revolutionized the treatment of hypercholesterolemia and cardiovascular diseases, in particular coronary heart disease. Initially thought to exert their effects through cholesterol reduction, recent evidence indicates that statins also have pleiotropic immunomodulatory properties independent of cholesterol lowering. In this review we will focus on the therapeutic applications and mechanisms involved in the MVA cascade including Rho GTPase and Rho kinase (ROCK) signaling, statin inhibition of HMGCR, geranylgeranyltransferase (GGTase) inhibition, and farnesyltransferase (FTase) inhibition in cardiovascular disease, pulmonary diseases (e.g. asthma and chronic obstructive pulmonary disease (COPD)), and cancer.
Keywords: Asthma; Farnesyl transferase inhibitors; Fibrosis; Geranylgeranyl transferase inhibitors; Rho GTPase; Statins.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors confirm that there is not any conflict of interest with individuals or organizations within three years of initiating the work that could inappropriately influence, or be perceived to influence, the study design or data interpretation.
Figures


References
-
- Abecassis I, Olofsson B, Schmid M, Zalcman G, Karniguian A. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res. 2003;291:363–376. - PubMed
-
- Adachi T, Vita R, Sannohe S, Stafford S, Alam R, Kayaba H, Chihara J. The functional role of rho and rho-associated coiled-coil forming protein kinase in eotaxin signaling of eosinophils. J Immunol. 2001;167:4609–4615. - PubMed
-
- Adiguzel E, Hou G, Sabatini PJ, Bendeck MP. Type VIII collagen signals via beta1 integrin and RhoA to regulate MMP-2 expression and smooth muscle cell migration. Matrix Biol. 2013 - PubMed
-
- Adnane J, Bizouarn FA, Qian Y, Hamilton AD, Sebti SM. p21(WAF1/CIP1) is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor beta- and Sp1-responsive element: involvement of the small GTPase rhoA. Molecular and cellular biology. 1998;18:6962–6970. - PMC - PubMed
-
- Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res. 2002;8:2225–2232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

