Improved poliovirus D-antigen yields by application of different Vero cell cultivation methods
- PMID: 24583004
- PMCID: PMC5355417
- DOI: 10.1016/j.vaccine.2014.02.022
Improved poliovirus D-antigen yields by application of different Vero cell cultivation methods
Abstract
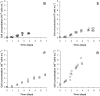
Vero cells were grown adherent to microcarriers (Cytodex 1; 3 g L(-1)) using animal component free media in stirred-tank type bioreactors. Different strategies for media refreshment, daily media replacement (semi-batch), continuous media replacement (perfusion) and recirculation of media, were compared with batch cultivation. Cell densities increased using a feed strategy from 1×10(6) cells mL(-1) during batch cultivation to 1.8, 2.7 and 5.0×10(6) cells mL(-1) during semi-batch, perfusion and recirculation, respectively. The effects of these different cell culture strategies on subsequent poliovirus production were investigated. Increased cell densities allowed up to 3 times higher D-antigen levels when compared with that obtained from batch-wise Vero cell culture. However, the cell specific D-antigen production was lower when cells were infected at higher cell densities. This cell density effect is in good agreement with observations for different cell lines and virus types. From the evaluated alternative culture methods, application of a semi-batch mode of operations allowed the highest cell specific D-antigen production. The increased product yields that can easily be reached using these higher cell density cultivation methods, showed the possibility for better use of bioreactor capacity for the manufacturing of polio vaccines to ultimately reduce vaccine cost per dose. Further, the use of animal-component-free cell- and virus culture media shows opportunities for modernization of human viral vaccine manufacturing.
Keywords: Adherent; Batch; Feed; Microcarriers; Perfusion; Recirculation.
Copyright © 2014. Published by Elsevier Ltd.
Figures
Similar articles
-
A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions.Vaccine. 2007 May 10;25(19):3879-89. doi: 10.1016/j.vaccine.2007.01.086. Epub 2007 Feb 5. Vaccine. 2007. PMID: 17307281
-
Adaptation of Vero cells to suspension growth for rabies virus production in different serum free media.Vaccine. 2019 Nov 8;37(47):6987-6995. doi: 10.1016/j.vaccine.2019.05.092. Epub 2019 Jun 11. Vaccine. 2019. PMID: 31201054
-
Development of suspension adapted Vero cell culture process technology for production of viral vaccines.Vaccine. 2019 Nov 8;37(47):6996-7002. doi: 10.1016/j.vaccine.2019.07.003. Epub 2019 Jul 6. Vaccine. 2019. PMID: 31288997
-
Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production.Appl Microbiol Biotechnol. 2016 Mar;100(5):2121-32. doi: 10.1007/s00253-015-7267-9. Epub 2016 Jan 13. Appl Microbiol Biotechnol. 2016. PMID: 26758296 Free PMC article. Review.
-
Bioreactor concepts for cell culture-based viral vaccine production.Expert Rev Vaccines. 2015;14(9):1181-95. doi: 10.1586/14760584.2015.1067144. Epub 2015 Jul 15. Expert Rev Vaccines. 2015. PMID: 26178380 Review.
Cited by
-
Vero cell upstream bioprocess development for the production of viral vectors and vaccines.Biotechnol Adv. 2020 Nov 15;44:107608. doi: 10.1016/j.biotechadv.2020.107608. Epub 2020 Aug 5. Biotechnol Adv. 2020. PMID: 32768520 Free PMC article. Review.
-
Production of Oncolytic Measles Virus in Vero Cells: Impact of Culture Medium and Multiplicity of Infection.Viruses. 2024 Nov 6;16(11):1740. doi: 10.3390/v16111740. Viruses. 2024. PMID: 39599854 Free PMC article.
-
Advances and challenges in poliomyelitis vaccines: a comprehensive review of development, production, and global deployment.Front Public Health. 2025 Jul 16;13:1611028. doi: 10.3389/fpubh.2025.1611028. eCollection 2025. Front Public Health. 2025. PMID: 40740383 Free PMC article. Review.
-
Production of a Foot-and-Mouth Disease Vaccine Antigen Using Suspension-Adapted BHK-21 Cells in a Bioreactor.Vaccines (Basel). 2021 May 13;9(5):505. doi: 10.3390/vaccines9050505. Vaccines (Basel). 2021. PMID: 34068378 Free PMC article.
-
Kinetic model for adherent Vero cell growth and poliovirus production in batch bioreactors.Process Biochem. 2019 Jun;81:156-164. doi: 10.1016/j.procbio.2019.03.010. Process Biochem. 2019. PMID: 31217725 Free PMC article.
References
-
- Bakker W.A.M., Thomassen Y.E., van’t Oever A.G., Westdijk J., van Oijen M.G.C.T., Sundermann L.C. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011;29(41):7188–7196. - PubMed
-
- Thomassen Y.E., van’t Oever A.G., Vinke M., Spiekstra A., Wijffels R.H., van der Pol L.A. Scale-down of the inactivated polio vaccine production process. Biotechnol Bioeng. 2013;110(5):1354–1365. - PubMed
-
- WHO Meeting of the strategic advisory group of experts on immunization. November 2012 – conclusions and recommendations. Wkly Epidemiol Rec. 2013;88(1):1–16. - PubMed
-
- Venczel L, Landry S, Aylward B, Sutter R, Sabow A, Smith G. Global post-eradication IPV supply and demand assessment: integrated findings commissioned by the Bill & Melinda Gates Foundation and prepared by Oliver Wyman Inc. Available online at: www.polioeradication.org. 2009, [accessed 16 September 2013].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

