Hyperglycemia induces abnormal gene expression in hematopoietic stem cells and their progeny in diabetic neuropathy
- PMID: 24583009
- PMCID: PMC4004028
- DOI: 10.1016/j.febslet.2014.02.030
Hyperglycemia induces abnormal gene expression in hematopoietic stem cells and their progeny in diabetic neuropathy
Abstract
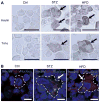
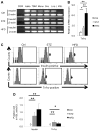
Diabetic peripheral neuropathy is a major chronic diabetic complication. We have previously shown that in type 1 diabetic streptozotocin-treated mice, insulin- and TNF-α co-expressing bone marrow-derived cells (BMDCs) induced by hyperglycemia travel to nerve tissues where they fuse with nerve cells, causing premature apoptosis and nerve dysfunction. Here we show that similar BMDCs also occur in type 2 diabetic high-fat diet (HFD) mice. Furthermore, we found that hyperglycemia induces the co-expression of insulin and TNF-α in c-kit(+)Sca-1(+)lineage(-) (KSL) progenitor cells, which maintain the same expression pattern in the progeny, which in turn participates in the fusion with neurons when transferred to normoglycemic animals.
Keywords: Cell–cell fusion; Hematopoietic stem cell; Hyperglycemia; Neuropathy; Stem cell abnormalities.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Malfunctioning CD106-positive, short-term hematopoietic stem cells trigger diabetic neuropathy in mice by cell fusion.Commun Biol. 2021 May 14;4(1):575. doi: 10.1038/s42003-021-02082-5. Commun Biol. 2021. PMID: 33990693 Free PMC article.
-
Bone marrow expression of poly(ADP-ribose) polymerase underlies diabetic neuropathy via hematopoietic-neuronal cell fusion.FASEB J. 2012 Jan;26(1):295-308. doi: 10.1096/fj.11-186262. Epub 2011 Oct 6. FASEB J. 2012. PMID: 21978940 Free PMC article.
-
Neuronal-Hematopoietic Cell Fusion in Diabetic Neuropathy.Stem Cells Transl Med. 2023 Apr 17;12(4):215-220. doi: 10.1093/stcltm/szad015. Stem Cells Transl Med. 2023. PMID: 36976582 Free PMC article.
-
Pathogenesis of diabetic neuropathy: bad to the bone.Ann N Y Acad Sci. 2011 Dec;1240:70-6. doi: 10.1111/j.1749-6632.2011.06309.x. Ann N Y Acad Sci. 2011. PMID: 22172042 Free PMC article. Review.
-
Peripheral Neuropathy in Mouse Models of Diabetes.Curr Protoc Mouse Biol. 2016 Sep 1;6(3):223-255. doi: 10.1002/cpmo.11. Curr Protoc Mouse Biol. 2016. PMID: 27584552 Free PMC article. Review.
Cited by
-
Malfunctioning CD106-positive, short-term hematopoietic stem cells trigger diabetic neuropathy in mice by cell fusion.Commun Biol. 2021 May 14;4(1):575. doi: 10.1038/s42003-021-02082-5. Commun Biol. 2021. PMID: 33990693 Free PMC article.
-
Bone Marrow-Derived Stem Cells: a Mixed Blessing in the Multifaceted World of Diabetic Complications.Curr Diab Rep. 2016 May;16(5):43. doi: 10.1007/s11892-016-0730-x. Curr Diab Rep. 2016. PMID: 27025211 Free PMC article. Review.
-
High Glucose-Induced PC12 Cell Death by Increasing Glutamate Production and Decreasing Methyl Group Metabolism.Biomed Res Int. 2016;2016:4125731. doi: 10.1155/2016/4125731. Epub 2016 Jun 19. Biomed Res Int. 2016. PMID: 27413747 Free PMC article.
-
Proinsulin-producing, hyperglycemia-induced adipose tissue macrophages underlie insulin resistance in high fat-fed diabetic mice.FASEB J. 2015 Aug;29(8):3537-48. doi: 10.1096/fj.15-271452. Epub 2015 May 7. FASEB J. 2015. PMID: 25953849 Free PMC article.
-
Islet adaptation to obesity and insulin resistance in WNIN/GR-Ob rats.Islets. 2014;6(5-6):e998099. doi: 10.1080/19382014.2014.998099. Islets. 2014. PMID: 25833252 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

