Past strategies and future directions for identifying AMP-activated protein kinase (AMPK) modulators
- PMID: 24583089
- PMCID: PMC3991011
- DOI: 10.1016/j.pharmthera.2014.02.008
Past strategies and future directions for identifying AMP-activated protein kinase (AMPK) modulators
Abstract
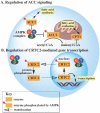
AMP-activated protein kinase (AMPK) is a promising therapeutic target for cancer, type II diabetes, and other illnesses characterized by abnormal energy utilization. During the last decade, numerous labs have published a range of methods for identifying novel AMPK modulators. The current understanding of AMPK structure and regulation, however, has propelled a paradigm shift in which many researchers now consider ADP to be an additional regulatory nucleotide of AMPK. How can the AMPK community apply this new understanding of AMPK signaling to translational research? Recent insights into AMPK structure, regulation, and holoenzyme-sensitive signaling may provide the hindsight needed to clearly evaluate the strengths and weaknesses of past AMPK drug discovery efforts. Improving future strategies for AMPK drug discovery will require pairing the current understanding of AMPK signaling with improved experimental designs.
Keywords: AMPK; Dephosphorylation inhibition; Drug discovery; High-throughput screening; Nucleotide analogs; Regulatory fragment.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965.J Biol Chem. 2018 Jun 8;293(23):8874-8885. doi: 10.1074/jbc.RA118.003547. Epub 2018 Apr 25. J Biol Chem. 2018. PMID: 29695504 Free PMC article.
-
Identifying New AMP-Activated Protein Kinase Inhibitors That Protect against Ischemic Brain Injury.ACS Chem Neurosci. 2019 May 15;10(5):2345-2354. doi: 10.1021/acschemneuro.8b00654. Epub 2019 Feb 22. ACS Chem Neurosci. 2019. PMID: 30763060
-
Probing the enzyme kinetics, allosteric modulation and activation of α1- and α2-subunit-containing AMP-activated protein kinase (AMPK) heterotrimeric complexes by pharmacological and physiological activators.Biochem J. 2016 Mar 1;473(5):581-92. doi: 10.1042/BJ20151051. Epub 2015 Dec 3. Biochem J. 2016. PMID: 26635351 Free PMC article.
-
Pharmacological Targeting of AMP-Activated Protein Kinase and Opportunities for Computer-Aided Drug Design.J Med Chem. 2016 Apr 14;59(7):2879-93. doi: 10.1021/acs.jmedchem.5b01201. Epub 2015 Nov 9. J Med Chem. 2016. PMID: 26510622 Review.
-
Screening methods for AMP-activated protein kinase modulators: a patent review.Expert Opin Ther Pat. 2015 Mar;25(3):261-77. doi: 10.1517/13543776.2014.995626. Epub 2014 Dec 23. Expert Opin Ther Pat. 2015. PMID: 25535089 Review.
Cited by
-
Substituted oxindol-3-ylidenes as AMP-activated protein kinase (AMPK) inhibitors.Eur J Med Chem. 2020 Jul 1;197:112316. doi: 10.1016/j.ejmech.2020.112316. Epub 2020 Apr 16. Eur J Med Chem. 2020. PMID: 32334266 Free PMC article.
-
LKB1 suppression promotes cardiomyocyte regeneration via LKB1-AMPK-YAP axis.Bosn J Basic Med Sci. 2022 Sep 16;22(5):772-783. doi: 10.17305/bjbms.2021.7225. Bosn J Basic Med Sci. 2022. PMID: 35490365 Free PMC article.
-
Inhibition of AMPK activation in Echinococcus granulosus sensu stricto limits the parasite's glucose metabolism and survival.Antimicrob Agents Chemother. 2024 Mar 6;68(3):e0120223. doi: 10.1128/aac.01202-23. Epub 2024 Feb 13. Antimicrob Agents Chemother. 2024. PMID: 38349157 Free PMC article.
-
The double-edged sword of AMPK signaling in cancer and its therapeutic implications.Arch Pharm Res. 2015 Mar;38(3):346-57. doi: 10.1007/s12272-015-0549-z. Epub 2015 Jan 10. Arch Pharm Res. 2015. PMID: 25575627 Free PMC article. Review.
-
Is 5´-AMP-Activated Protein Kinase Both Jekyll and Hyde in Bladder Cancer?Int Neurourol J. 2015 Jun;19(2):55-66. doi: 10.5213/inj.2015.19.2.55. Epub 2015 Jun 29. Int Neurourol J. 2015. PMID: 26126434 Free PMC article. Review.
References
-
- Anderson SN, Cool BL, Kifle L, Chiou W, Egan DA, Barrett LW, Richardson PL, Frevert EU, Warrior U, Kofron JL, Burns DJ. Microarrayed compound screening (microARCS) to identify activators and inhibitors of AMP-activated protein kinase. J Biomol Screen. 2004;9:112–121. - PubMed
-
- Azevedo R, van Zeeland M, Raaijmakers H, Kazemier B, de Vlieg J, Oubrie A. X-ray structure of p38alpha bound to TAK-715: comparison with three classic inhibitors. Acta Crystallogr D Biol Crystallogr. 2012;68:1041–1050. - PubMed
-
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5’-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. - PubMed
-
- Burwinkel B, Scott JW, Buhrer C, van Landeghem FK, Cox GF, Wilson CJ, Grahame Hardie D, Kilimann MW. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

