A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting
- PMID: 24583183
- PMCID: PMC4089096
- DOI: 10.1016/j.ijpara.2014.01.010
A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting
Abstract
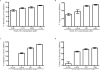
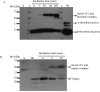
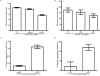
Ixodes scapularis is a medically important tick species that transmits causative agents of important human tick-borne diseases including borreliosis, anaplasmosis and babesiosis. An understanding of how this tick feeds is needed prior to the development of novel methods to protect the human population against tick-borne disease infections. This study characterizes a blood meal-induced I. scapularis (Ixsc) tick saliva serine protease inhibitor (serpin (S)), in-house referred to as IxscS-1E1. The hypothesis that ticks use serpins to evade the host's defense response to tick feeding is based on the assumption that tick serpins inhibit functions of protease mediators of the host's anti-tick defense response. Thus, it is significant that consistent with hallmark characteristics of inhibitory serpins, Pichia pastoris-expressed recombinant IxscS-1E1 (rIxscS-1E1) can trap thrombin and trypsin in SDS- and heat-stable complexes, and reduce the activity of the two proteases in a dose-responsive manner. Additionally, rIxscS-1E1 also inhibited, but did not apparently form detectable complexes with, cathepsin G and factor Xa. Our data also show that rIxscS-1E1 may not inhibit chymotrypsin, kallikrein, chymase, plasmin, elastase and papain even at a much higher rIxscS-1E1 concentration. Native IxscS-1E1 potentially plays a role(s) in facilitating I. scapularis tick evasion of the host's hemostatic defense as revealed by the ability of rIxscS-1E1 to inhibit adenosine diphosphate- and thrombin-activated platelet aggregation, and delay activated partial prothrombin time and thrombin time plasma clotting in a dose-responsive manner. We conclude that native IxscS-1E1 is part of the tick saliva protein complex that mediates its anti-hemostatic, and potentially inflammatory, functions by inhibiting the actions of thrombin, trypsin and other yet unknown trypsin-like proteases at the tick-host interface.
Keywords: Ixodes scapularis; Serine protease inhibitors; Tick anti-blood clotting function; Tick anti-platelet aggregation function; Tick-feeding physiology.
Copyright © 2014 Australian Society for Parasitology Inc. Published by Elsevier Ltd. All rights reserved.
Figures

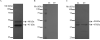
Similar articles
-
The putative role of Rhipicephalus microplus salivary serpins in the tick-host relationship.Insect Biochem Mol Biol. 2016 Apr;71:12-28. doi: 10.1016/j.ibmb.2016.01.004. Epub 2016 Feb 2. Insect Biochem Mol Biol. 2016. PMID: 26844868 Free PMC article.
-
Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions.Int J Parasitol. 2015 Aug;45(9-10):613-27. doi: 10.1016/j.ijpara.2015.03.009. Epub 2015 May 5. Int J Parasitol. 2015. PMID: 25957161 Free PMC article.
-
Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation.Insect Mol Biol. 2013 Jun;22(3):306-19. doi: 10.1111/imb.12024. Epub 2013 Mar 24. Insect Mol Biol. 2013. PMID: 23521000 Free PMC article.
-
Novel secretory vesicle serpins, endopin 1 and endopin 2: endogenous protease inhibitors with distinct target protease specificities.Biol Chem. 2002 Jul-Aug;383(7-8):1067-74. doi: 10.1515/BC.2002.115. Biol Chem. 2002. PMID: 12437089 Review.
-
Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction.Front Cell Infect Microbiol. 2017 May 29;7:216. doi: 10.3389/fcimb.2017.00216. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28611951 Free PMC article. Review.
Cited by
-
Heparan sulfate/heparin glycosaminoglycan binding alters inhibitory profile and enhances anticoagulant function of conserved Amblyomma americanum tick saliva serpin 19.Insect Biochem Mol Biol. 2017 Jan;80:1-10. doi: 10.1016/j.ibmb.2016.11.002. Epub 2016 Nov 12. Insect Biochem Mol Biol. 2017. PMID: 27845251 Free PMC article.
-
Inhibition of bovine platelets aggregation in response to Hyalomma anatolicum salivary gland proteins/peptides.Vet World. 2016 Nov;9(11):1264-1268. doi: 10.14202/vetworld.2016.1264-1268. Epub 2016 Nov 17. Vet World. 2016. PMID: 27956779 Free PMC article.
-
Amblyomma americanum serpin 41 (AAS41) inhibits inflammation by targeting chymase and chymotrypsin.Int J Biol Macromol. 2020 Aug 1;156:1007-1021. doi: 10.1016/j.ijbiomac.2020.04.088. Epub 2020 Apr 19. Int J Biol Macromol. 2020. PMID: 32320803 Free PMC article.
-
Iripin-3, a New Salivary Protein Isolated From Ixodes ricinus Ticks, Displays Immunomodulatory and Anti-Hemostatic Properties In Vitro.Front Immunol. 2021 Mar 1;12:626200. doi: 10.3389/fimmu.2021.626200. eCollection 2021. Front Immunol. 2021. PMID: 33732248 Free PMC article.
-
A 24-48 h fed Amblyomma americanum tick saliva immuno-proteome.BMC Genomics. 2014 Jun 24;15:518. doi: 10.1186/1471-2164-15-518. BMC Genomics. 2014. PMID: 24962723 Free PMC article.
References
-
- Askew DJ, Askew YS, Kato Y, Luke CJ, Pak SC, Brömme D, Silverman GA. The amplified mouse squamous cell carcinoma antigen gene locus contains a serpin (Serpinb3b) that inhibits both papain-like cysteine and trypsin-like serine proteinases. Genomics. 2004;84:166–175. - PubMed
-
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983;308:740–742. - PubMed
-
- Berger BW, Clemmensen OJ, Ackerman AB. Lyme disease is a spirochetosis. Am. J. Dermatopathol. 1983;5:115–124. - PubMed
-
- Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

