Chromatin dynamics: interplay between remodeling enzymes and histone modifications
- PMID: 24583555
- PMCID: PMC4099280
- DOI: 10.1016/j.bbagrm.2014.02.013
Chromatin dynamics: interplay between remodeling enzymes and histone modifications
Abstract
Chromatin dynamics play an essential role in regulating the accessibility of genomic DNA for a variety of nuclear processes, including gene transcription and DNA repair. The posttranslational modification of the core histones and the action of ATP-dependent chromatin remodeling enzymes represent two primary mechanisms by which chromatin dynamics are controlled and linked to nuclear events. Although there are examples in which a histone modification or a remodeling enzyme may be sufficient to drive a chromatin transition, these mechanisms typically work in concert to integrate regulatory inputs, leading to a coordinated alteration in chromatin structure and function. Indeed, site-specific histone modifications can facilitate the recruitment of chromatin remodeling enzymes to particular genomic regions, or they can regulate the efficiency or the outcome of a chromatin remodeling reaction. Conversely, chromatin remodeling enzymes can also influence, and sometimes directly modulate, the modification state of histones. These functional interactions are generally complex, frequently transient, and often require the association of myriad additional factors. This article is part of a Special Issue entitled: Molecular mechanisms of histone modification function.
Keywords: Chromatin dynamics; Chromatin remodeling; Histone modifications.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
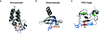
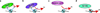
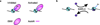
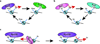
References
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. - PubMed
-
- Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

