G0/G1 switch gene 2 has a critical role in adipocyte differentiation
- PMID: 24583640
- PMCID: PMC4207475
- DOI: 10.1038/cdd.2014.26
G0/G1 switch gene 2 has a critical role in adipocyte differentiation
Abstract
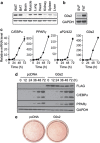
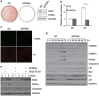
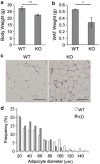
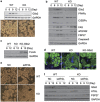
Mouse 3T3-L1 preadipocytes differentiate into adipocytes when treated with 3-isobutyl-1-methylxanthine, dexamethasone, and insulin. Although mechanisms of adipogenesis, including transcriptional cascades, are understood, it is still unclear how clonally expanded cells eventually enter the terminal differentiation program. From gene expression profile studies, we identified G0/G1 switch gene 2 (G0s2) as a novel regulator of adipogenesis. The gene was found to be expressed at a higher level in white and brown adipose tissues, and it was induced in 3T3-L1 cells by hormonal treatment. Importantly, G0s2 expression was closely associated with the transition from mitotic clonal expansion to terminal differentiation. Knockdown of G0s2 expression with siRNA inhibited adipocyte differentiation, whereas constitutive overexpression of G0s2 accelerated differentiation of preadipocytes to mature adipocytes. Expression of G0s2 was found to be regulated by peroxisome proliferator-activated receptor γ (PPARγ), which is a well-known regulator of adipocyte differentiation. Absence of either PPARγ or G0s2 expression resulted in apoptotic pathway activation before terminal differentiation. To determine whether G0s2 has a role in vivo, G0s2-knockout mice were generated. The knockout mice were normal in appearance, but they had less adipose mass than wild-type littermates. Mouse embryonic fibroblast cells from G0s2-deficient mice exhibited impaired adipogenesis and contained unusually small intracellular lipid droplets, suggesting that G0s2 has a role in lipid droplet formation. Our studies demonstrate that G0s2 has an important role in adipogenesis and accumulation of triacylglycerol.
Figures



Similar articles
-
Palmitate induces fat accumulation by activating C/EBPβ-mediated G0S2 expression in HepG2 cells.World J Gastroenterol. 2017 Nov 21;23(43):7705-7715. doi: 10.3748/wjg.v23.i43.7705. World J Gastroenterol. 2017. PMID: 29209111 Free PMC article.
-
TNF-α reduces g0s2 expression and stimulates lipolysis through PPAR-γ inhibition in 3T3-L1 adipocytes.Cytokine. 2014 Oct;69(2):196-205. doi: 10.1016/j.cyto.2014.06.005. Epub 2014 Jul 1. Cytokine. 2014. PMID: 24993166
-
Porcine G₀/G₁ switch gene 2 (G0S2) expression is regulated during adipogenesis and short-term in-vivo nutritional interventions.Lipids. 2013 Mar;48(3):209-18. doi: 10.1007/s11745-013-3756-8. Epub 2013 Jan 16. Lipids. 2013. PMID: 23322075
-
Fat Cell and Fatty Acid Turnover in Obesity.Adv Exp Med Biol. 2017;960:135-160. doi: 10.1007/978-3-319-48382-5_6. Adv Exp Med Biol. 2017. PMID: 28585198 Review.
-
Untapped Pharmaceutical Potential of 4,5,4'-Trihydroxy-3,3'-dimethoxybibenzyl for Regulating Obesity: A Cell-Based Study with a Focus on Terminal Differentiation in Adipogenesis.J Nat Prod. 2022 Jun 24;85(6):1591-1602. doi: 10.1021/acs.jnatprod.2c00213. Epub 2022 Jun 9. J Nat Prod. 2022. PMID: 35679136 Review.
Cited by
-
Cocktail supplement with rosiglitazone: a novel inducer for chicken preadipocyte differentiation in vitro.Biosci Rep. 2016 Nov 3;36(6):e00401. doi: 10.1042/BSR20160049. Print 2016 Dec. Biosci Rep. 2016. PMID: 27638500 Free PMC article.
-
Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice.Am J Physiol Lung Cell Mol Physiol. 2014 Oct 15;307(8):L618-31. doi: 10.1152/ajplung.00144.2014. Epub 2014 Aug 22. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25150063 Free PMC article.
-
Gastric Cancer Growth Modulated by circSNTB2/miR-6938-5p/G0S2 and PDCD4.Comb Chem High Throughput Screen. 2023;26(11):1990-2002. doi: 10.2174/1386207326666221108112113. Comb Chem High Throughput Screen. 2023. PMID: 36366842 Free PMC article.
-
Crucial role of iron in epigenetic rewriting during adipocyte differentiation mediated by JMJD1A and TET2 activity.Nucleic Acids Res. 2023 Jul 7;51(12):6120-6142. doi: 10.1093/nar/gkad342. Nucleic Acids Res. 2023. PMID: 37158274 Free PMC article.
-
G0S2 represses PI3K/mTOR signaling and increases sensitivity to PI3K/mTOR pathway inhibitors in breast cancer.Cell Cycle. 2017;16(21):2146-2155. doi: 10.1080/15384101.2017.1371884. Epub 2017 Sep 14. Cell Cycle. 2017. PMID: 28910567 Free PMC article.
References
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. - PubMed
-
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. - PubMed
-
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. - PubMed
-
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. - PubMed
-
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

