Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo
- PMID: 24583641
- PMCID: PMC4013513
- DOI: 10.1038/cdd.2014.15
Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo
Abstract
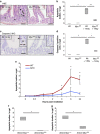
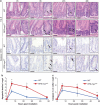
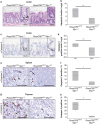
Recent studies have suggested that C-MYC may be an excellent therapeutic cancer target and a number of new agents targeting C-MYC are in preclinical development. Given most therapeutic regimes would combine C-MYC inhibition with genotoxic damage, it is important to assess the importance of C-MYC function for DNA damage signalling in vivo. In this study, we have conditionally deleted the c-Myc gene in the adult murine intestine and investigated the apoptotic response of intestinal enterocytes to DNA damage. Remarkably, c-Myc deletion completely abrogated the immediate wave of apoptosis following both ionizing irradiation and cisplatin treatment, recapitulating the phenotype of p53 deficiency in the intestine. Consistent with this, c-Myc-deficient intestinal enterocytes did not upregulate p53. Mechanistically, this was linked to an upregulation of the E3 Ubiquitin ligase Mdm2, which targets p53 for degradation in c-Myc-deficient intestinal enterocytes. Further, low level overexpression of c-Myc, which does not impact on basal levels of apoptosis, elicited sustained apoptosis in response to DNA damage, suggesting c-Myc activity acts as a crucial cell survival rheostat following DNA damage. We also identify the importance of MYC during DNA damage-induced apoptosis in several other tissues, including the thymus and spleen, using systemic deletion of c-Myc throughout the adult mouse. Together, we have elucidated for the first time in vivo an essential role for endogenous c-Myc in signalling DNA damage-induced apoptosis through the control of the p53 tumour suppressor protein.
Figures



References
-
- Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. - PubMed
-
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. - PubMed
-
- Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. - PubMed
-
- Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. - PubMed
-
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

