Development and evaluation of a new immunohistochemistry-based test for the detection of rabies virus neutralizing antibodies
- PMID: 24583787
- PMCID: PMC4896518
- DOI: 10.4161/hv.28042
Development and evaluation of a new immunohistochemistry-based test for the detection of rabies virus neutralizing antibodies
Abstract
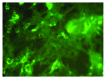
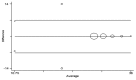
Rabies claims about 55,000 human lives and many hundreds of thousands of livestock every year, worldwide. Despite a heavy disease burden, laboratory facilities to diagnose the infection remain scarce in most countries of the developing world where the disease is endemic. Rapid Fluorescent Focus Inhibition Test (RFFIT) and Fluorescent Antibody Virus Neutralization Test (FAVN) are the common tests done in the rabies diagnostic laboratories to detect and quantitate Rabies Virus Neutralizing Antibodies (RVNA). RFFIT is most often employed in confirming seroconversion following prophylactic vaccination, and to aid ante-mortem diagnosis in suspected cases of rabies. Though this remains one of the most sought-after diagnostic services in rabies laboratories, the requirements for expensive anti-rabies fluorochrome antibody conjugate and a fluorescent microscope restrict its performance to only a few reference laboratories. Cost-effective laboratory diagnostic methods employing affordable technology are a need of the hour in the rabies-endemic countries. In this study we have developed a new immunohistochemistry-based neutralization test and extensively evaluated it along with RFFIT. One hundred and 20 human serum samples collected after post-exposure vaccination were subjected to both the tests for determining RVNA titers. The results obtained with the new test correlated significantly with those of RFFIT. Further validation of the inter- and intra- assay precision, lower limit of quantification (LLOQ) and specificity was also performed. The best correlation between the 2 methods, however, was observed only when the RVNA concentrations in the samples were>20 IU/mL. Overall, the immunohistrochemistry-based neutralization test yielded satisfactory results. We suggest that it might serve as a cost-effective alternative to RFFIT in low-resource settings in the developing countries.
Keywords: RFFIT; immunohistochemistry; post-exposure prophylaxis; rabies; rabies virus neutralizing antibodies.
Figures


Similar articles
-
Development and evaluation of an anti-rabies virus phosphoprotein-specific monoclonal antibody for detection of rabies neutralizing antibodies using RFFIT.PLoS Negl Trop Dis. 2017 Dec 21;11(12):e0006084. doi: 10.1371/journal.pntd.0006084. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29267277 Free PMC article.
-
Rapid fluorescent focus inhibition test optimization and validation: Improved detection of neutralizing antibodies to rabies virus.J Immunol Methods. 2019 Nov;474:112626. doi: 10.1016/j.jim.2019.06.017. Epub 2019 Jun 20. J Immunol Methods. 2019. PMID: 31228423
-
A new recombinant rabies virus expressing a green fluorescent protein: A novel and fast approach to quantify virus neutralizing antibodies.Biologicals. 2019 May;59:56-61. doi: 10.1016/j.biologicals.2019.03.002. Epub 2019 Mar 18. Biologicals. 2019. PMID: 30898479
-
A comparative review of serological assays for the detection of rabies virus-specific antibodies.Acta Trop. 2022 Feb;226:106254. doi: 10.1016/j.actatropica.2021.106254. Epub 2021 Nov 20. Acta Trop. 2022. PMID: 34808119 Review.
-
Review of Detection and Quantification of Rabies Virus Antibodies.Viral Immunol. 2021 Oct;34(8):522-530. doi: 10.1089/vim.2020.0317. Epub 2021 Sep 22. Viral Immunol. 2021. PMID: 34550784 Review.
Cited by
-
Bites from the same dog, different outcomes for two patients: a case report.Infect Dis Poverty. 2017 Jul 5;6(1):107. doi: 10.1186/s40249-017-0321-3. Infect Dis Poverty. 2017. PMID: 28676127 Free PMC article.
-
Exploring one health-based strategies for rabies elimination: Overview and future prospects.PLoS Negl Trop Dis. 2025 Jun 18;19(6):e0013159. doi: 10.1371/journal.pntd.0013159. eCollection 2025 Jun. PLoS Negl Trop Dis. 2025. PMID: 40531916 Free PMC article. Review.
-
Differential Host Immune Responses after Infection with Wild-Type or Lab-Attenuated Rabies Viruses in Dogs.PLoS Negl Trop Dis. 2015 Aug 20;9(8):e0004023. doi: 10.1371/journal.pntd.0004023. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26292099 Free PMC article.
-
Co-culture: A quick approach for isolation of street rabies virus in murine neuroblastoma cells.Vet World. 2015 May;8(5):636-9. doi: 10.14202/vetworld.2015.636-639. Epub 2015 May 21. Vet World. 2015. PMID: 27047148 Free PMC article.
References
-
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath Narayana DH, Abdul Rahman S, Meslin F-, Lobo D, Ravikumar K, Gangaboraiah. . Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis 2007; 11:29 - 35; http://dx.doi.org/10.1016/j.ijid.2005.10.007; PMID: 16678463 - DOI - PubMed
-
- Nicholson KG. Cell culture vaccines for human use. General considerations. In: Meslin FX, Koprowsky H and Kaplan MM. (eds.). Laboratory techniques in Rabies. 4th ed. Geneva, WHO, 1996, pp.271-9.
-
- Smith SJ, Yager AP, Baer MG. Rapid Fluorescent Focus Inhibition Test for determining rabies virus neutralizing antibodies. In: Meslin FX, Kaplan MM, Koprowski H (eds.). Laboratory techniques in Rabies. 4th ed. Geneva: WHO, 1996, pp. 181-91.
-
- Cliquet F, Aubert M, Sagne L. . Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies virus neutralising antibody. J Immunol Methods 1998; 212:79 - 87; http://dx.doi.org/10.1016/S0022-1759(97)00212-3; PMID: 9671155 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
