Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors
- PMID: 24583800
- PMCID: PMC3990645
- DOI: 10.1158/1078-0432.CCR-13-0279
Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors
Abstract
Janus-activated kinases (JAK) are the mediators of a variety of cytokine signals via their cognate receptors that result in activation of intracellular signaling pathways. Alterations in JAK1, JAK2, JAK3, and TYK2 signaling contribute to different disease states, and dysregulated JAK-STAT signaling is associated with hematologic malignancies, autoimmune disorders, and immune-deficient conditions. Genetic alterations of JAK2 occur in the majority of patients with myeloproliferative neoplasms and occur in a subset of patients with acute leukemias. JAK-mediated signaling critically relies on STAT transcription factors, and on activation of the MAPK and PI3K/Akt signaling axes. Hyperactive JAK at the apex of these potent oncogenic signaling pathways therefore represents an important target for small-molecule kinase inhibitors in different disease states. The JAK1/2 inhibitor ruxolitinib and the JAK3 inhibitor tofacitinib were recently approved for the treatment of myelofibrosis and rheumatoid arthritis, respectively, and additional ATP-competitive JAK inhibitors are in clinical development. Although these agents show clinical activity, the ability of these JAK inhibitors to induce clinical/molecular remissions in hematologic malignancies seems limited and resistance upon chronic drug exposure is seen. Alternative modes of targeting JAK2 such as allosteric kinase inhibition or HSP90 inhibition are under evaluation, as is the use of histone deacetylase inhibitors. Combination therapy approaches integrating inhibition of STAT, PI3K/Akt, and MAPK pathways with JAK kinase inhibitors might be critical to overcome malignancies characterized by dysregulated JAK signaling.
©2014 AACR.
Conflict of interest statement
Conflicts of interest: none
Figures
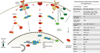
References
-
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. - PubMed
-
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
-
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

