Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells
- PMID: 24583847
- PMCID: PMC3975354
- DOI: 10.3390/ijms15033560
Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells
Abstract
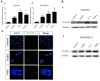
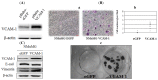
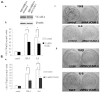
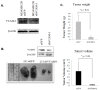
VCAM-1 (CD106), a transmembrane glycoprotein, was first reported to play an important role in leukocyte adhesion, leukocyte transendothelial migration and cell activation by binding to integrin VLA-1 (α4β1). In the present study, we observed that VCAM-1 expression can be induced in many breast cancer epithelial cells by cytokine stimulation in vitro and its up-regulation directly correlated with advanced clinical breast cancer stage. We found that VCAM-1 over-expression in the NMuMG breast epithelial cells controls the epithelial and mesenchymal transition (EMT) program to increase cell motility rates and promote chemoresistance to doxorubicin and cisplatin in vitro. Conversely, in the established MDAMB231 metastatic breast cancer cell line, we confirmed that knockdown of endogenous VCAM-1 expression reduced cell proliferation and inhibited TGFβ1 or IL-6 mediated cell migration, and increased chemosensitivity. Furthermore, we demonstrated that knockdown of endogenous VCAM-1 expression in MDAMB231 cells reduced tumor formation in a SCID xenograft mouse model. Signaling studies showed that VCAM-1 physically associates with CD44 and enhances CD44 and ABCG2 expression. Our findings uncover the possible mechanism of VCAM-1 activation facilitating breast cancer progression, and suggest that targeting VCAM-1 is an attractive strategy for therapeutic intervention.
Figures

Similar articles
-
Overexpression of CD44 accompanies acquired tamoxifen resistance in MCF7 cells and augments their sensitivity to the stromal factors, heregulin and hyaluronan.BMC Cancer. 2012 Oct 6;12:458. doi: 10.1186/1471-2407-12-458. BMC Cancer. 2012. PMID: 23039365 Free PMC article.
-
High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion.Oncotarget. 2013 Jan;4(1):48-63. doi: 10.18632/oncotarget.756. Oncotarget. 2013. PMID: 23295955 Free PMC article.
-
The role of a new CD44st in increasing the invasion capability of the human breast cancer cell line MCF-7.BMC Cancer. 2011 Jul 12;11:290. doi: 10.1186/1471-2407-11-290. BMC Cancer. 2011. PMID: 21749678 Free PMC article.
-
Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1.Cell Oncol (Dordr). 2017 Jun;40(3):199-208. doi: 10.1007/s13402-017-0324-x. Epub 2017 May 22. Cell Oncol (Dordr). 2017. PMID: 28534212 Review.
-
Ectopic Tumor VCAM-1 Expression in Cancer Metastasis and Therapy Resistance.Cells. 2022 Dec 4;11(23):3922. doi: 10.3390/cells11233922. Cells. 2022. PMID: 36497180 Free PMC article. Review.
Cited by
-
Cisplatin-resistant triple-negative breast cancer subtypes: multiple mechanisms of resistance.BMC Cancer. 2019 Nov 4;19(1):1039. doi: 10.1186/s12885-019-6278-9. BMC Cancer. 2019. PMID: 31684899 Free PMC article.
-
Epithelial-Mesenchymal Plasticity in Organotropism Metastasis and Tumor Immune Escape.J Clin Med. 2019 May 25;8(5):747. doi: 10.3390/jcm8050747. J Clin Med. 2019. PMID: 31130637 Free PMC article. Review.
-
MCPIP3 as a Potential Metastasis Suppressor Gene in Human Colorectal Cancer.Int J Mol Sci. 2018 May 3;19(5):1350. doi: 10.3390/ijms19051350. Int J Mol Sci. 2018. PMID: 29751537 Free PMC article.
-
Cancer cells remodel themselves and vasculature to overcome the endothelial barrier.Cancer Lett. 2016 Oct 1;380(2):534-544. doi: 10.1016/j.canlet.2014.10.031. Epub 2014 Oct 31. Cancer Lett. 2016. PMID: 25449784 Free PMC article. Review.
-
Prioritization of drug targets for thyroid cancer: a multi-omics Mendelian randomization study.Endocrine. 2024 Nov;86(2):732-743. doi: 10.1007/s12020-024-03933-x. Epub 2024 Jun 19. Endocrine. 2024. PMID: 38896366
References
-
- Hartge P. Abortion breast cancer and epidemiology. N. Engl. J. Med. 1997;336:127–128. - PubMed
-
- Mettlin C.J., Menck H.R., Winchester D.P., Murphy G.P. A comparison of breast colorectal lung and prostate cancers reported to the National Cancer Data Base and the Surveillance Epidemiology and End Results Program. Cancer. 1997;79:2052–2061. - PubMed
-
- Bostner J., Ahnstrom-Waltersson M., Fornander T., Skoog L., Nordenskjold B., Stal O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26:6997–7005. - PubMed
-
- Hui R., Campbell D.H., Lee C.S., McCaul K., Horsfall D.J., Musgrove E.A., Daly R.J., Seshadri R., Sutherland R.L. EMS1 amplification can occur independently of CCND1 or INT-2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–1623. - PubMed
-
- Mukherjee S., Conrad S.E. c-Myc suppresses p21WAF1/CIP1 expression during estrogen signaling and antiestrogen resistance in human breast cancer cells. J. Biol. Chem. 2005;280:17617–17625. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

