MgCaCO3 versus CaCO3 in peritoneal dialysis patients--a cross-over pilot trial
- PMID: 24584605
- PMCID: PMC4335925
- DOI: 10.3747/pdi.2013.00129
MgCaCO3 versus CaCO3 in peritoneal dialysis patients--a cross-over pilot trial
Abstract
Background: Despite adverse effects such as constipation, vascular calcification, and hypercalcemia, calcium-based salts are relatively affordable and effective phosphate binders that remain in widespread use in the dialysis population. We conducted a pilot study examining whether the use of a combined magnesium/calcium-based binder was as effective as calcium carbonate at lowering serum phosphate levels in peritoneal dialysis (PD) patients.
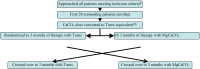
Methods: This was a cross-over, investigator-masked pilot study in which prevalent PD patients received calcium carbonate alone (200 mg calcium per tablet) or calcium magnesium carbonate (100 mg calcium, 85 mg magnesium per tablet). Primary outcome was serum phosphate level at 3 months. Analysis was as per protocol.
Results: Twenty patients were recruited, 17 completed the study. Mean starting dose was 11.35 ± 7.04 pills per day of MgCaCO3 and 9.00 ± 4.97 pills per day of CaCO3. Mean phosphate levels fell from 2.13 mmol/L to 2.01 mmol/L (95% confidence interval (CI): 1.76 - 2.30, p = 0.361) in the MgCaCO3 group, and 1.81 mmol/L (95% CI: 1.56 - 2.0, p = 0.026) in the CaCO3 alone group. Six (35%) patients taking MgCaCO3 and 9 (54%) taking CaCO3 alone achieved Kidney Disease Outcomes Quality Initiative (KDOQI) serum phosphate targets at 3 months. Diarrhea developed in 9 patients taking MgCaCO3 and 3 taking CaCO3. Serum magnesium exceeded 1.4 mmol/L in 5 patients taking MgCaCO3 while serum calcium exceeded 2.65 mmol/L in 3 patients receiving CaCO3. When compared to the initial dose, the prescribed dose at 3 months was reduced by 44% (to 6.41 tablets/day) in the MgCaCO3 group and by 8% (to 8.24 pills per day) in the CaCO3 alone group.
Conclusion: Compared with CaCO3 alone, the preparation and dose of MgCaCO3 used in this pilot study was no better at lowering serum phosphate levels in PD patients, and was associated with more dose-limiting side effects.
Keywords: Phosphate-binders; adverse effects; compliance rates; magnesium calcium carbonate.
Copyright © 2015 International Society for Peritoneal Dialysis.
Figures

References
-
- Emmett M. A comparison of clinically useful phosphorus binders for patients with chronic kidney failure. Kidney Int 2004; 66:S25–32. - PubMed
-
- de Francisco ALM, Leidig M, Covic AC, Ketteler M, Benedyk-Lorens E, Mircescu GM, et al. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant 2010; 25(11):3707–17. - PMC - PubMed
-
- Parsons V, Baldwin D, Moniz C, Marsden J, Ball E, Rifkin I. Successful control of hyperparathyroidism in patients on continuous ambulatory peritoneal dialysis using magnesium carbonate and calcium carbonate as phosphate binders. Nephron 1993; 63(4):379–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

