Predicting the Peritoneal Absorption of Icodextrin in Rats and Humans Including the Effect of α-Amylase Activity in Dialysate
- PMID: 24584610
- PMCID: PMC4443987
- DOI: 10.3747/pdi.2012.00247
Predicting the Peritoneal Absorption of Icodextrin in Rats and Humans Including the Effect of α-Amylase Activity in Dialysate
Abstract
Background: Contrary to ultrafiltration, the three-pore model predictions of icodextrin absorption from the peritoneal cavity have not yet been reported likely, in part, due to difficulties in estimating the degradation of glucose-polymer chains by α-amylase activity in dialysate. We incorporated this degradation process in a modified three-pore model of peritoneal transport to predict ultrafiltration and icodextrin absorption simultaneously in rats and humans.
Methods: Separate three-pore models were constructed for humans and rats. The model for humans was adapted from PD Adequest 2.0 including a clearance term out of the peritoneal cavity to account for the absorption of large molecules to the peritoneal tissues, and considering patients who routinely used icodextrin by establishing steady-state plasma concentrations. The model for rats employed a standard three-pore model in which human kinetic parameters were scaled for a rat based on differences in body weight. Both models described the icodextrin molecular weight (MW) distribution as five distinct MW fractions. First order kinetics was applied using degradation rate constants obtained from previous in-vitro measurements using gel permeation chromatography. Ultrafiltration and absorption were predicted during a 4-hour exchange in rats, and 9 and 14-hour exchanges in humans with slow to fast transport characteristics with and without the effect of amylase activity.
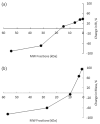
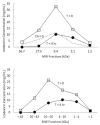
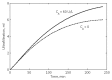
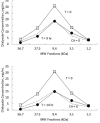
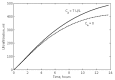
Results: In rats, the icodextrin MW profile shifted towards the low MW fractions due to complete disappearance of the MW fractions greater than 27.5 kDa. Including the effect of amylase activity (60 U/L) resulted in 21.1% increase in ultrafiltration (UF) (7.6 mL vs 6.0 mL) and 7.1% increase in icodextrin absorption (CHO) (62.5% with vs 58.1%). In humans, the shift in MW profile was less pronounced. The fast transport (H) patient absorbed more icodextrin than the slow transport (L) patient during both 14-hour (H: 47.9% vs L: 40.2%) and 9-hour (H: 37.4% vs L: 31.7%) exchanges. While the UF was higher during the longer exchange, it varied modestly among the patient types (14-hour range: 460 - 509 mL vs 9-hour range: 382 - 389 mL). When averaged over all patients, the increases in UF and CHO during the 14-hour exchange due to amylase activity (7 U/L) were 15% and 1.5%, respectively.
Conclusion: The icodextrin absorption values predicted by the model agreed with those measured in rats and humans to accurately show the increased absorption in rats. Also, the model confirmed the previous suggestions by predicting an increase in UF specific to amylase activity in dialysate, likely due to the added osmolality by the small molecules generated as a result of the degradation process. As expected, this increase was more pronounced in rats than in humans because of higher dialysate concentrations of amylase in rats.
Keywords: Icodextrin; absorption; amylase activity; ultrafiltration.
Copyright © 2015 International Society for Peritoneal Dialysis.
Figures

Similar articles
-
Modelling of icodextrin hydrolysis and kinetics during peritoneal dialysis.Sci Rep. 2023 Apr 21;13(1):6526. doi: 10.1038/s41598-023-33480-w. Sci Rep. 2023. PMID: 37085652 Free PMC article.
-
Icodextrin metabolism and alpha-amylase activity in nonuremic rats undergoing chronic peritoneal dialysis.Perit Dial Int. 2007 Jul-Aug;27(4):415-23. Perit Dial Int. 2007. PMID: 17602150
-
Automated peritoneal dialysis prescriptions for enhancing sodium and fluid removal: a predictive analysis of optimized, patient-specific dwell times for the day period.Perit Dial Int. 2013 Nov-Dec;33(6):646-54. doi: 10.3747/pdi.2012.00261. Perit Dial Int. 2013. PMID: 24335125 Free PMC article.
-
Icodextrin: a review of its use in peritoneal dialysis.Drugs. 2003;63(19):2079-105. doi: 10.2165/00003495-200363190-00011. Drugs. 2003. PMID: 12962523 Review.
-
Peritoneal transport with icodextrin solution.Contrib Nephrol. 2006;150:97-103. doi: 10.1159/000093508. Contrib Nephrol. 2006. PMID: 16720998 Review.
Cited by
-
Can one long peritoneal dwell with icodextrin replace two short dwells with glucose?Front Physiol. 2024 Jul 10;15:1339762. doi: 10.3389/fphys.2024.1339762. eCollection 2024. Front Physiol. 2024. PMID: 39050480 Free PMC article.
-
Icodextrin Simplifies PD Therapy by Equalizing UF and Sodium Removal Among Patient Transport Types During Long Dwells: A Modeling Study.Perit Dial Int. 2016 Jan-Feb;36(1):79-84. doi: 10.3747/pdi.2013.00081. Epub 2014 Sep 2. Perit Dial Int. 2016. PMID: 25185017 Free PMC article.
-
Modelling of icodextrin hydrolysis and kinetics during peritoneal dialysis.Sci Rep. 2023 Apr 21;13(1):6526. doi: 10.1038/s41598-023-33480-w. Sci Rep. 2023. PMID: 37085652 Free PMC article.
-
Mechanisms of Crystalloid versus Colloid Osmosis across the Peritoneal Membrane.J Am Soc Nephrol. 2018 Jul;29(7):1875-1886. doi: 10.1681/ASN.2017080828. Epub 2018 May 29. J Am Soc Nephrol. 2018. PMID: 29844208 Free PMC article.
-
Comparing Computational Peritoneal Dialysis Models in Pigs and Patients.Toxins (Basel). 2025 Jun 28;17(7):329. doi: 10.3390/toxins17070329. Toxins (Basel). 2025. PMID: 40711140 Free PMC article.
References
-
- Rippe G, Levin L. Computer simulation of ultrafiltration profiles for an icodextrin-based fluid in CAPD. Kidney Int 2000; 57:2546–56. - PubMed
-
- Vonesh EF, Story KO, Douma CE, Krediet RT. Modeling of icodextrin in PD Adequest 2.0. Perit Dial Int 2006; 26:475–81. - PubMed
-
- Galach M, Werynski A, Waniewski J, Freida P, Lindholm B. Kinetic analysis of peritoneal fluid and solute transport with combination of glucose and icodextrin as osmotic agents. Perit Dial Int 2009; 29:72–80. - PubMed
-
- Akonur A, Leypoldt JK. Three-pore model predictions of 24-hour therapy using bimodal solutions. Perit Dial Int 2011; 31:537–44. - PubMed
-
- Rippe A, Rippe C, Sward K, Rippe B. Disproportionally low clearance of macromolecules from the plasma to the peritoneal cavity in a mouse model of peritoneal dialysis. Nephrol Dial Transplant 2007; 22:88–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

