The importance of Purkinje activation in long duration ventricular fibrillation
- PMID: 24584738
- PMCID: PMC3959715
- DOI: 10.1161/JAHA.113.000495
The importance of Purkinje activation in long duration ventricular fibrillation
Abstract
Background: The mechanisms that maintain long duration ventricular fibrillation (LDVF) are unclear. The difference in distribution of the Purkinje system in dogs and pigs was explored to determine if Purkinje activation propagates to stimulate working myocardium (WM) during LDVF and WM pacing.
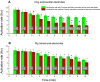
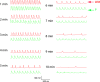
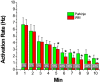
Methods and results: In-vivo extracellular recordings were made from 1044 intramural plunge and epicardial plaque electrodes in 6 pig and 6 dog hearts. Sinus activation propagated sequentially from the endocardium to the epicardium in dogs but not pigs. During epicardial pacing, activation propagated along the endocardium and traversed the LV wall almost parallel to the epicardium in dogs, but in pigs propagated away from the pacing site approximately perpendicular to the epicardium. After 1 minute of VF, activation rate near the endocardium was significantly faster than near the epicardium in dogs (P<0.01) but not pigs (P>0.05). From 2 to 10 minutes of LDVF, recordings exhibiting Purkinje activations were near the endocardium in dogs (P<0.01) but were scattered transmurally in pigs, and the WM activation rate in recordings in which Purkinje activations were present was significantly faster than the WM activation rate in recordings in which Purkinje activations were absent (P<0.01). In 10 isolated perfused dog hearts, the LV endocardium was exposed and 2 microelectrodes were inserted into Purkinje and adjacent myocardial cells. After 5 minutes of LDVF, mean Purkinje activation rate was significantly faster than mean WM activation rate (P<0.01).
Conclusion: These extracellular and intracellular findings about activation support the hypothesis that Purkinje activation propagates to stimulate WM during sinus rhythm, pacing, and LDVF.
Keywords: arrhythmia (mechanisms); long duration ventricular fibrillation; ventricular arrhythmia.
Figures






Similar articles
-
Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H883-9. doi: 10.1152/ajpheart.00466.2008. Epub 2008 Jun 27. Am J Physiol Heart Circ Physiol. 2008. PMID: 18586887 Free PMC article.
-
Endocardial focal activation originating from Purkinje fibers plays a role in the maintenance of long duration ventricular fibrillation.Croat Med J. 2014 Apr;55(2):121-7. doi: 10.3325/cmj.2014.55.121. Croat Med J. 2014. PMID: 24778098 Free PMC article.
-
Intramural optical mapping of V(m) and Ca(i)2+ during long-duration ventricular fibrillation in canine hearts.Am J Physiol Heart Circ Physiol. 2012 Mar 15;302(6):H1294-305. doi: 10.1152/ajpheart.00426.2011. Epub 2012 Jan 20. Am J Physiol Heart Circ Physiol. 2012. PMID: 22268104 Free PMC article.
-
The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation.J Cardiovasc Electrophysiol. 2007 Dec;18(12):1306-12. doi: 10.1111/j.1540-8167.2007.00963.x. Epub 2007 Oct 4. J Cardiovasc Electrophysiol. 2007. PMID: 17916154
-
Mechanisms of Long-Duration Ventricular Fibrillation in Human Hearts and Experimental Validation in Canine Purkinje Fibers.JACC Clin Electrophysiol. 2015 Jun;1(3):187-197. doi: 10.1016/j.jacep.2015.04.003. Epub 2015 Apr 20. JACC Clin Electrophysiol. 2015. PMID: 29759364
Cited by
-
2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias.J Interv Card Electrophysiol. 2020 Oct;59(1):145-298. doi: 10.1007/s10840-019-00663-3. J Interv Card Electrophysiol. 2020. PMID: 31984466 Free PMC article.
-
Image-Based Structural Modeling of the Cardiac Purkinje Network.Biomed Res Int. 2015;2015:621034. doi: 10.1155/2015/621034. Epub 2015 Oct 25. Biomed Res Int. 2015. PMID: 26583120 Free PMC article.
-
Two-to-one Purkinje-to-myocardium activation during ventricular fibrillation associated with hypertrophic cardiomyopathy.HeartRhythm Case Rep. 2022 Dec 6;9(2):113-117. doi: 10.1016/j.hrcr.2022.11.013. eCollection 2023 Feb. HeartRhythm Case Rep. 2022. PMID: 36860756 Free PMC article. No abstract available.
-
Effects of combination of sotalol and verapamil on initiation, maintenance, and termination of ventricular fibrillation in swine hearts.Cardiovasc Ther. 2018 Jun;36(3):e12326. doi: 10.1111/1755-5922.12326. Epub 2018 Mar 25. Cardiovasc Ther. 2018. PMID: 29485248 Free PMC article.
-
2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias.Europace. 2019 Aug 1;21(8):1143-1144. doi: 10.1093/europace/euz132. Europace. 2019. PMID: 31075787 Free PMC article.
References
-
- Huang J, Rogers JM, Killingsworth CR, Singh KP, Smith WM, Ideker RE. Evolution of activation patterns during long‐duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2004; 286:H1193-H1200 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

