The GTPase-deficient Rab27A(Q78L) mutant inhibits melanosome transport in melanocytes through trapping of Rab27A effector protein Slac2-a/melanophilin in their cytosol: development of a novel melanosome-targetinG tag
- PMID: 24584932
- PMCID: PMC4036246
- DOI: 10.1074/jbc.M114.552281
The GTPase-deficient Rab27A(Q78L) mutant inhibits melanosome transport in melanocytes through trapping of Rab27A effector protein Slac2-a/melanophilin in their cytosol: development of a novel melanosome-targetinG tag
Abstract
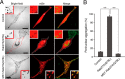
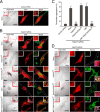
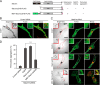
The small GTPase Rab27A is a crucial regulator of actin-based melanosome transport in melanocytes, and functionally defective Rab27A causes human Griscelli syndrome type 2, which is characterized by silvery hair. A GTPase-deficient, constitutively active Rab27A(Q78L) mutant has been shown to act as an inhibitor of melanosome transport and to induce perinuclear aggregation of melanosomes, but the molecular mechanism by which Rab27A(Q78L) inhibits melanosome transport remained to be determined. In this study, we attempted to identify the primary cause of the perinuclear melanosome aggregation induced by Rab27A(Q78L). The results showed that Rab27A(Q78L) is unable to localize on mature melanosomes and that its inhibitory activity on melanosome transport is completely dependent on its binding to the Rab27A effector Slac2-a/melanophilin. When we forcibly expressed Rab27A(Q78L) on mature melanosomes by using a novel melanosome-targeting tag that we developed in this study and named the MST tag, the MST-Rab27A(Q78L) fusion protein behaved in the same manner as wild-type Rab27A. It localized on mature melanosomes without inducing melanosome aggregation and restored normal peripheral melanosome distribution in Rab27A-deficient cells. These findings indicate that the GTPase activity of Rab27A is required for its melanosome localization but is not required for melanosome transport.
Keywords: Low Molecular Weight G Proteins; Melanogenesis; Membrane Trafficking; Protein Targeting; Rab; Small GTPases; Subcellular Organelles.
Figures



Similar articles
-
The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes.Mol Cell Biol. 2003 Aug;23(15):5245-55. doi: 10.1128/MCB.23.15.5245-5255.2003. Mol Cell Biol. 2003. PMID: 12861011 Free PMC article.
-
Functional analysis of Slac2-c/MyRIP as a linker protein between melanosomes and myosin VIIa.J Biol Chem. 2005 Jul 29;280(30):28015-22. doi: 10.1074/jbc.M501465200. Epub 2005 May 30. J Biol Chem. 2005. PMID: 15927964
-
Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome.J Biol Chem. 2003 Mar 28;278(13):11386-92. doi: 10.1074/jbc.M211996200. Epub 2003 Jan 16. J Biol Chem. 2003. PMID: 12531900
-
Evidence for defective Rab GTPase-dependent cargo traffic in immune disorders.Exp Cell Res. 2013 Sep 10;319(15):2360-7. doi: 10.1016/j.yexcr.2013.06.012. Epub 2013 Jun 26. Exp Cell Res. 2013. PMID: 23810987 Free PMC article. Review.
-
Rab GTPases: Key players in melanosome biogenesis, transport, and transfer.Pigment Cell Melanoma Res. 2021 Mar;34(2):222-235. doi: 10.1111/pcmr.12931. Epub 2020 Oct 14. Pigment Cell Melanoma Res. 2021. PMID: 32997883 Review.
Cited by
-
The adaptor protein melanophilin regulates dynamic myosin-Va:cargo interaction and dendrite development in melanocytes.Mol Biol Cell. 2019 Mar 15;30(6):742-752. doi: 10.1091/mbc.E18-04-0237. Epub 2019 Jan 30. Mol Biol Cell. 2019. PMID: 30699046 Free PMC article.
-
How myosin VI traps its off-state, is activated and dimerizes.Nat Commun. 2023 Oct 23;14(1):6732. doi: 10.1038/s41467-023-42376-2. Nat Commun. 2023. PMID: 37872146 Free PMC article.
-
AID-2×RBD27, an auxin-inducible degron-based Rab27 trapper that reversibly inhibits the function of Rab27A in melanocytes.J Cell Sci. 2025 Jun 1;138(11):jcs263878. doi: 10.1242/jcs.263878. Epub 2025 Jun 9. J Cell Sci. 2025. PMID: 40351074 Free PMC article.
-
Exosome-derived microRNAs: emerging players in vitiligo.Front Immunol. 2024 Jul 8;15:1419660. doi: 10.3389/fimmu.2024.1419660. eCollection 2024. Front Immunol. 2024. PMID: 39040109 Free PMC article. Review.
-
A Comprehensive Review of Mammalian Pigmentation: Paving the Way for Innovative Hair Colour-Changing Cosmetics.Biology (Basel). 2023 Feb 11;12(2):290. doi: 10.3390/biology12020290. Biology (Basel). 2023. PMID: 36829566 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

