Phage lysis: three steps, three choices, one outcome
- PMID: 24585055
- PMCID: PMC4012431
- DOI: 10.1007/s12275-014-4087-z
Phage lysis: three steps, three choices, one outcome
Abstract
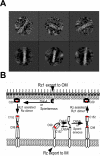
The lysis of bacterial hosts by double-strand DNA bacteriophages, once thought to reflect merely the accumulation of sufficient lysozyme activity during the infection cycle, has been revealed to recently been revealed to be a carefully regulated and temporally scheduled process. For phages of Gramnegative hosts, there are three steps, corresponding to subversion of each of the three layers of the cell envelope: inner membrane, peptidoglycan, and outer membrane. The pathway is controlled at the level of the cytoplasmic membrane. In canonical lysis, a phage encoded protein, the holin, accumulates harmlessly in the cytoplasmic membrane until triggering at an allele-specific time to form micron-scale holes. This allows the soluble endolysin to escape from the cytoplasm to degrade the peptidoglycan. Recently a parallel pathway has been elucidated in which a different type of holin, the pinholin, which, instead of triggering to form large holes, triggers to form small, heptameric channels that serve to depolarize the membrane. Pinholins are associated with SAR endolysins, which accumulate in the periplasm as inactive, membrane-tethered enzymes. Pinholin triggering collapses the proton motive force, allowing the SAR endolysins to refold to an active form and attack the peptidoglycan. Surprisingly, a third step, the disruption of the outer membrane is also required. This is usually achieved by a spanin complex, consisting of a small outer membrane lipoprotein and an integral cytoplasmic membrane protein, designated as o-spanin and i-spanin, respectively. Without spanin function, lysis is blocked and progeny virions are trapped in dead spherical cells, suggesting that the outer membrane has considerable tensile strength. In addition to two-component spanins, there are some single-component spanins, or u-spanins, that have an N-terminal outer-membrane lipoprotein signal and a C-terminal transmembrane domain. A possible mechanism for spanin function to disrupt the outer membrane is to catalyze fusion of the inner and outer membranes.
Figures




Similar articles
-
Phage Lysis: Multiple Genes for Multiple Barriers.Adv Virus Res. 2019;103:33-70. doi: 10.1016/bs.aivir.2018.09.003. Epub 2018 Nov 28. Adv Virus Res. 2019. PMID: 30635077 Free PMC article. Review.
-
Identifying components of the Shewanella phage LambdaSo lysis system.J Bacteriol. 2024 Jun 20;206(6):e0002224. doi: 10.1128/jb.00022-24. Epub 2024 May 21. J Bacteriol. 2024. PMID: 38771038 Free PMC article.
-
[Lysis of bacterial cells in the process of bacteriophage release--canonical and newly discovered mechanisms].Postepy Hig Med Dosw (Online). 2015 Jan 23;69:114-26. Postepy Hig Med Dosw (Online). 2015. PMID: 25614679 Review. Polish.
-
Endolysin Regulation in Phage Mu Lysis.mBio. 2022 Jun 28;13(3):e0081322. doi: 10.1128/mbio.00813-22. Epub 2022 Apr 26. mBio. 2022. PMID: 35471081 Free PMC article.
-
Localization and Regulation of the T1 Unimolecular Spanin.J Virol. 2018 Oct 29;92(22):e00380-18. doi: 10.1128/JVI.00380-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30135120 Free PMC article.
Cited by
-
Genomic Characterization of Two Shiga Toxin-Converting Bacteriophages Induced From Environmental Shiga Toxin-Producing Escherichia coli.Front Microbiol. 2021 Feb 25;12:587696. doi: 10.3389/fmicb.2021.587696. eCollection 2021. Front Microbiol. 2021. PMID: 33716997 Free PMC article.
-
Biochemical characterisation and production kinetics of high molecular-weight (HMW) putative antibacterial proteins of insect pathogenic Brevibacillus laterosporus isolates.BMC Microbiol. 2024 Jul 13;24(1):259. doi: 10.1186/s12866-024-03340-2. BMC Microbiol. 2024. PMID: 38997685 Free PMC article.
-
Diversity of Salmonella enterica phages isolated from chicken farms in Kenya.Microbiol Spectr. 2024 Jan 11;12(1):e0272923. doi: 10.1128/spectrum.02729-23. Epub 2023 Dec 11. Microbiol Spectr. 2024. PMID: 38078723 Free PMC article.
-
Imaging the Infection Cycle of T7 at the Single Virion Level.Int J Mol Sci. 2022 Sep 24;23(19):11252. doi: 10.3390/ijms231911252. Int J Mol Sci. 2022. PMID: 36232552 Free PMC article.
-
Genomic characterization of a new phage BUCT541 against Klebsiella pneumoniae K1-ST23 and efficacy assessment in mouse and Galleria mellonella larvae.Front Microbiol. 2022 Sep 16;13:950737. doi: 10.3389/fmicb.2022.950737. eCollection 2022. Front Microbiol. 2022. PMID: 36187954 Free PMC article.
References
-
- Adhya S, Sen A, Mitra S. In: The role of gene S In: The Bacteriophage Lambda. Hershey AD, editor. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1971. pp. 743–746.
-
- Atkins JF, Steitz JA, Anderson CW, Model P. Binding of mammalian ribosomes to MS2 phage RNA reveals an overlapping gene encoding a lysis function. Cell. 1979;18:247–256. - PubMed
-
- Barenboim M, Chang CY, dib Hajj F, Young R. Characterization of the dual start motif of a class II holin gene. Mol.Microbiol. 1999;32:715–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

