Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia
- PMID: 24585095
- PMCID: PMC6904104
- DOI: 10.3892/ijo.2014.2313
Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia
Abstract
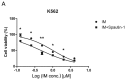
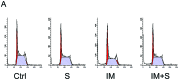
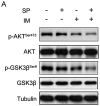
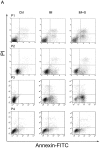
Imatinib mesylate (IM), a targeted competitive inhibitor of the BCR-ABL tyrosine kinase, has revolutionized the clinical treatment of chronic myeloid leukemia (CML). However, resistance and intolerance are still a challenge in the treatment of CML. Autophagy has been proposed to play a role in IM resistance. To investigate the anti-leukemic activity of specific and potent autophagy inhibitor-1 (spautin-1) in CML, we detected its synergistic effect with IM in K562 and CML cells. Our results showed that spautin-1 markedly inhibited IM-induced autophagy in CML cells by downregulating Beclin-1. Spautin-1 enhanced IM-induced CML cell apoptosis by reducing the expression of the anti-apoptotic proteins Mcl-1 and Bcl-2. We further demonstrated that the pro-apoptotic activity of spautin-1 was associated with activation of GSK3β, an important downstream effector of PI3K/AKT. The findings indicate that the autophagy inhibitor spautin-1 enhances IM-induced apoptosis by inactivating PI3K/AKT and activating downstream GSK3β, leading to downregulation of Mcl-1 and Bcl-2, which represents a promising approach to improve the efficacy of IM in the treatment of patients with CML.
Figures












Similar articles
-
Divalproex sodium enhances the anti-leukemic effects of imatinib in chronic myeloid leukemia cells partly through SIRT1.Cancer Lett. 2015 Jan 28;356(2 Pt B):791-9. doi: 10.1016/j.canlet.2014.10.033. Epub 2014 Oct 31. Cancer Lett. 2015. PMID: 25449787
-
Targeting of GSK3β promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells.Blood. 2012 Mar 8;119(10):2335-45. doi: 10.1182/blood-2011-06-361261. Epub 2012 Jan 18. Blood. 2012. PMID: 22262776
-
Induction of heme oxygenase-1 by Na+-H+ exchanger 1 protein plays a crucial role in imatinib-resistant chronic myeloid leukemia cells.J Biol Chem. 2015 May 15;290(20):12558-71. doi: 10.1074/jbc.M114.626960. Epub 2015 Mar 23. J Biol Chem. 2015. PMID: 25802333 Free PMC article. Clinical Trial.
-
Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells.Leukemia. 2012 Aug;26(8):1752-60. doi: 10.1038/leu.2012.65. Epub 2012 Mar 7. Leukemia. 2012. PMID: 22395361
-
Characterization of cancer stem cells in chronic myeloid leukaemia.Biochem Soc Trans. 2007 Nov;35(Pt 5):1347-51. doi: 10.1042/BST0351347. Biochem Soc Trans. 2007. PMID: 17956348 Review.
Cited by
-
Recent advances in understanding chronic myeloid leukemia: where do we stand?Fac Rev. 2021 Apr 1;10:35. doi: 10.12703/r/10-35. eCollection 2021. Fac Rev. 2021. PMID: 33977288 Free PMC article. Review.
-
Tricyclic antidepressant amitriptyline inhibits autophagic flux and prevents tube formation in vascular endothelial cells.Basic Clin Pharmacol Toxicol. 2019 Apr;124(4):370-384. doi: 10.1111/bcpt.13146. Epub 2018 Nov 15. Basic Clin Pharmacol Toxicol. 2019. PMID: 30311396 Free PMC article.
-
Autophagy induced by Helicobacter Pylori infection can lead to gastric cancer dormancy, metastasis, and recurrence: new insights.Hum Cell. 2024 Jan;37(1):139-153. doi: 10.1007/s13577-023-00996-2. Epub 2023 Nov 4. Hum Cell. 2024. PMID: 37924488 Review.
-
Cell Plasticity and Prostate Cancer: The Role of Epithelial-Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance.Cancers (Basel). 2021 Jun 4;13(11):2795. doi: 10.3390/cancers13112795. Cancers (Basel). 2021. PMID: 34199763 Free PMC article. Review.
-
Autophagy-Related Deubiquitinating Enzymes Involved in Health and Disease.Cells. 2015 Oct 5;4(4):596-621. doi: 10.3390/cells4040596. Cells. 2015. PMID: 26445063 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

