Bronchial thermoplasty for moderate or severe persistent asthma in adults
- PMID: 24585221
- PMCID: PMC6986472
- DOI: 10.1002/14651858.CD009910.pub2
Bronchial thermoplasty for moderate or severe persistent asthma in adults
Abstract
Background: Bronchial thermoplasty is a procedure that consists of the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. It has been suggested that bronchial thermoplasty works by reducing airway smooth muscle, thereby reducing the ability of the smooth muscle to bronchoconstrict. This treatment could then reduce asthma symptoms and exacerbations, resulting in improved asthma control and quality of life.
Objectives: To determine the efficacy and safety of bronchial thermoplasty in adults with bronchial asthma.
Search methods: We searched the Cochrane Airways Group Specialised Register of Trials (CAGR) up to January 2014.
Selection criteria: We included randomised controlled clinical trials that compared bronchial thermoplasty versus any active control in adults with moderate or severe persistent asthma. Our primary outcomes were quality of life, asthma exacerbations and adverse events.
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias.
Main results: We included three trials (429 participants) with differences regarding their design (two trials compared bronchial thermoplasty vs medical management and the other compared bronchial thermoplasty vs a sham intervention) and participant characteristics; one of the studies included participants with more symptomatic asthma compared with the others.The pooled analysis showed improvement in quality of life at 12 months in participants who received bronchial thermoplasty that did not reach the threshold for clinical significance (3 trials, 429 participants; mean difference (MD) in Asthma Quality of Life Questionnaire (AQLQ) scores 0.28, 95% confidence interval (CI) 0.07 to 0.50; moderate-quality evidence). Measures of symptom control showed no significant differences (3 trials, 429 participants; MD in Asthma Control Questionnaire (ACQ) scores -0.15, 95% CI -0.40 to 0.10; moderate-quality evidence). The risk of bias for these outcomes was high because two of the studies did not have a sham intervention for the control group.The results from two trials showed a lower rate of exacerbation after 12 months of treatment for participants who underwent bronchial thermoplasty. The trial with sham intervention showed a significant reduction in the proportion of participants visiting the emergency department for respiratory symptoms, from 15.3% on sham treatment to 8.4% over 12 months following thermoplasty. The trials showed no significant improvement in pulmonary function parameters (with the exception of a greater increase in morning peak expiratory flow (PEF) in one trial). Treated participants who underwent bronchial thermoplasty had a greater risk of hospitalisation for respiratory adverse events during the treatment period (3 trials, 429 participants; risk ratio 3.50, 95% CI 1.26 to 9.68; high-quality evidence), which represents an absolute increase from 2% to 8% (95% CI 3% to 23%) over the treatment period. This means that six of 100 participants treated with thermoplasty (95% CI 1 to 21) would require an additional hospitalisation over the treatment period. No significant difference in the risk of hospitalisation was noted at the end of the treatment period.Bronchial thermoplasty was associated with an increase in respiratory adverse events, mainly during the treatment period. Most of these events were mild or moderate, appeared in the 24-hour post-treatment period, and were resolved within a week.
Authors' conclusions: Bronchial thermoplasty for patients with moderate to severe asthma provides a modest clinical benefit in quality of life and lower rates of asthma exacerbation, but no significant difference in asthma control scores. The quality of life findings are at risk of bias, as the main benefits were seen in the two studies that did not include a sham treatment arm. This procedure increases the risk of adverse events during treatment but has a reasonable safety profile after completion of the bronchoscopies. The overall quality of evidence regarding this procedure is moderate. For clinical practice, it would be advisable to collect data from patients systematically in independent clinical registries. Further research should provide better understanding of the mechanisms of action of bronchial thermoplasty, as well as its effect in different asthma phenotypes or in patients with worse lung function.
Conflict of interest statement
AT: Received payment for lectures including service on speakers bureaus GSK, Astra Zeneca, Esteve, Prodesfarma, Pfizer, Chiesi. Furthermore AT is a member of a Scientific Steering Committee of an international registry of patients treated with bronchial thermoplasty. He works at the Respiratory Department of the Hospital de la Santa Creu I Sant Pau, and since October 12 performs bronchial thermoplasty in patients with severe asthma.
IS: None known
AMM: None known
MRiF: None known
JJY‐N: None known
PAC: None known
VP: Recieved payment for lectures including service on speakers bureaus from NOvartis, AstraZeneca and Merck and as a consultant for Almirall. He also received payment for development of educational presentations from AstraZeneca, Chiesi and Amgem and travel/accommodations/meeting expenses unrelated to activities listed from AstraZeneca and Merck.
Figures
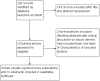






Update of
References
References to studies included in this review
AIR {published data only}
-
- Cox G, Laviolette M, Rubin A, Corris P, Niven R, Thomson N, et al. 5‐Year safety of bronchial thermoplasty demonstrated in patients with moderate to severe asthma: Asthma Intervention Research (AIR) trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6839.
-
- Cox G, Miller J, Rubin A, Corris P, Thomson N, Niven R, et al. Asthma intervention research (AIR) trial evaluating bronchial thermoplasty: early results [Abstract]. American Thoracic Society International Conference; 2006 May 19‐21; San Diego. 2006:A711;Poster A45.
-
- Cox G, Miller JD, Rubin AS, Corris P, Thomson NC, Niven R, et al. Impact of bronchial thermoplasty on asthma status interim results from the AIR trial [Abstract]. European Respiratory Journal 2006;28(Suppl 50):114s;Abstract 749.
-
- Cox G, Rubin A, Laviolette M, Pavord I, Thomson N. Efficacy of bronchial thermoplasty (BT) in patients with severe asthma: the AIR2 trial [Abstract]. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:3044.
-
- Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. The New England Journal of Medicine 2007;356(13):1327‐37. [PUBMED: 17392302] - PubMed
AIR 2 {published data only}
-
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Annals of Allergy, Asthma & Immunology 2011;107(1):65‐70. [PUBMED: 21704887] - PubMed
-
- Castro M, Rubin AS, Laviolette M, Fiterman J, Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double‐blind, sham‐controlled clinical trial. American Journal of Respiratory and Critical Care Medicine 2010;181(2):116‐24. [PUBMED: 19815809] - PMC - PubMed
-
- Castro M, Rubin AS, Laviolette M, Hanania NA, Cox G. Two‐year persistence of effect of bronchial thermoplasty (BT) in patients with severe asthma: AIR2 Trial [Abstract]. Chest 2010;138(4):768A.
-
- Castro M, Rubin AS, Laviolette M, Thomson NC. Efficacy of bronchial thermoplasty (BT) in patients with severe asthma: the AIR2 trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A3644.
-
- Shah P, Fiterman J, McEvoy C, Erzurum S, AIR2. Safety of bronchial thermoplasty (BT) in patients with severe, symptomatic asthma: positive safety profile in the AIR2 trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A2814; Poster #J82.
RISA {published data only}
-
- Pavord I, Laviolette M, Thomson N, Niven R, Cox G, Corris P. 5‐Year Safety Of Bronchial Thermoplasty Demonstrated In Patients With Severe Refractory Asthma: Research In Severe Asthma (RISA) Trial. American Thoracic Society International Conference; 2011 May 13‐18; Denver. 2011; Vol. 183:A6382.
-
- Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. American Journal of Respiratory and Critical Care Medicine 2007;176(12):1185‐91. [PUBMED: 17901415] - PubMed
References to studies excluded from this review
Hales 2010 {published data only}
-
- Hales JB, Wechsler ME, Niven R, Prys‐Picard C, Castro M, Cox G. Patient selection for bronchial thermoplasty (BT) following FDA approval: lessons learned from multiple clinical trials [Abstract]. Annals of Allergy, Asthma and Immunology 2010;105(5 Suppl):Not provided.
Wechsler 2009 {published data only}
-
- Wechsler M, Cox G, Niven R, Zoratti E, Hanania N. Where could bronchial thermoplasty (BT) fit into your allergy practice? Lessons learned from multiple BT clinical trials [Abstract]. Annual Scientific Meeting of the American College of Allergy, Asthma and Immunology; 2009 Nov 5‐10; Miami. 2009.
Additional references
Bricknell 2012
-
- Bicknell S, Chaudhuri R, Shepherd M, Lee N, Pitman N, Spears M, et al. Introducing bronchial thermoplasty treatment into a severe asthma clinical service. Thorax 2012;67:A65‐A66. [DOI: 10.1136/thoraxjnl-2012-202678.146] - DOI
Brown 2005
-
- Brown RH, Wizeman W, Danek C, Mitzner W. In vivo evaluation of the effectiveness of bronchial thermoplasty with computed tomography. Journal of Applied Physiology 2005;98(5):1603‐6. [PUBMED: 15718404] - PubMed
Cayetano 2011
-
- Cayetano KS, Chan AL, Albertson TE, Yoneda KY. Bronchial thermoplasty: a new treatment paradigm for severe persistent asthma. Clinical Reviews in Allergy and Immunology 2011 Nov 22 [Epub ahead of print]. [PUBMED: 22105704] - PubMed
Danek 2004
-
- Danek CJ, Lombard CM, Dungworth DL, Cox PG, Miller JD, Biggs MJ, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. Journal of Applied Physiology 2004;97(5):1946‐53. [PUBMED: 15258133] - PubMed
Doeing 2013
Egger 1997
GINA 2011
-
- Global Strategy for Asthma Management, Prevention. Global Initiative for Asthma (GINA) guidelines 2011. www.ginasthma.org/ (accessed 11 April 2012).
Gordon 2013
-
- Gordon IO, Husain AN, Charbeneau J, Krishnan JA, Hogarth DK. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. The Journal of Asthma 2013;50(6):634‐41. [PUBMED: 23621125] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jones 2002
-
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [PUBMED: 11936514] - PubMed
Juniper 1994
-
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47(1):81‐7. [PUBMED: 8283197] - PubMed
Juniper 1999
-
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. The European Respiratory Journal 1999;14(4):902‐7. [PUBMED: 10573240] - PubMed
Mayse 2007
-
- Mayse M, Laviolette M, Rubin A, Lampron N, Simoff M, Duhamel D, et al. Clinical pearls for bronchial thermoplasty. How I do it. Journal of Bronchology 2007;14:115‐23.
Miller 2005
-
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005;127(6):1999‐2006. [PUBMED: 15947312] - PubMed
Moore 2006
-
- Moore WC, Peters SP. Severe asthma: an overview. Journal of Allergy and Clinical Immunology 2006;117(3):487‐94. [PUBMED: 16522445] - PubMed
NCT01350336
-
- Bronchial Thermoplasty in Severe Persistent Asthma (PAS2). http://clinicaltrials.gov/ct2/show/NCT01350336 (accessed 7 May 2013). [NCT01350336]
Pavord 2011
-
- Pavord I, Laviolette M, Thomson N, Niven R, Cox G, Corris P, et al. 5‐Year safety of bronchial thermoplasty demonstrated in patients with severe refractory asthma: Research In Severe Asthma (RISA) trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A6382.
Pavord 2013
-
- Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. for the Research in Severe Asthma Trial Study Group. Safety of bronchial thermoplasty in patients with severe refractory asthma. Annals of Allergy, Asthma & Immunology 2013;111(5):402‐7. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rodriguez‐Trigo 2008
-
- Rodríguez‐Trigo G, Plaza V, Picado C, Sanchis J. Management according to the Global Initiative for Asthma Guidelines with near‐fatal asthma reduces morbidity and mortality. Archivos de Bronconeumologia 2008;44:192‐6. - PubMed
Taylor 2008
-
- Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. European Respiratory Journal 2008;32(3):545‐54. [PUBMED: 18757695] - PubMed
Thomson 2011
Torrego 2010
-
- Torrego Fernández, A. Bronchial thermoplasty in the treatment of asthma. Archivos de Bronconeumologia 2010;46(2):85‐91. - PubMed
Wahidi 2012
-
- Wahidi MM, Kraft M. Bronchial thermoplasty for severe asthma. American Journal of Respiratory and Critical Care Medicine 2012;185(7):709‐14. [PUBMED: 22077066] - PubMed
Wechsler 2013
-
- Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa E Silva JR, Shah PL, et al. for the Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long‐term safety and effectiveness in patients with severe persistent asthma. Journal of Allergy and Clinical Immunology. 2013 Aug 30. pii: S0091‐6749(13)01268‐2. [DOI: 10.1016/j.jaci.2013.08.009; PUBMED: 23998657] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

