Modified silicone elastomer vaginal gels for sustained release of antiretroviral HIV microbicides
- PMID: 24585370
- PMCID: PMC3989844
- DOI: 10.1002/jps.23913
Modified silicone elastomer vaginal gels for sustained release of antiretroviral HIV microbicides
Abstract
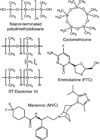
We previously reported nonaqueous silicone elastomer gels (SEGs) for sustained vaginal administration of the CCR5-targeted entry inhibitor maraviroc (MVC). Here, we describe chemically modified SEGs (h-SEGs) in which the hydrophobic cyclomethicone component was partially replaced with relatively hydrophilic silanol-terminated polydimethylsiloxanes (st-PDMS). MVC and emtricitabine (a nucleoside reverse transcriptase inhibitor), both currently under evaluation as topical microbicides to counter sexual transmission of human immunodeficiency virus type 1 (HIV-1), were used as model antiretroviral (ARV) drugs. Gel viscosity and in vitro ARV release were significantly influenced by st-PDMS molecular weight and concentration in the h-SEGs. Unexpectedly, gels prepared with lower molecular weight grades of st-PDMS showed higher viscosities. h-SEGs provided enhanced release over 24 h compared with aqueous hydroxyethylcellulose (HEC) gels, did not modify the pH of simulated vaginal fluid (SVF), and were shown to less cytotoxic than standard HEC vaginal gel. ARV solubility increased as st-PDMS molecular weight decreased (i.e., as percentage hydroxyl content increased), helping to explain the in vitro release trends. Dye ingression and SVF dilution studies confirmed the increased hydrophilicity of the h-SEGs. h-SEGs have potential for use in vaginal drug delivery, particularly for ARV-based HIV-1 microbicides.
Keywords: HIV/AIDS; HIVmicrobicides; antiinfectives; drug delivery systems; formulation; gels; rheology; silicone elastomer; sustained release; viscosity.
© 2014 Wiley Periodicals, Inc. and the American Pharmacists Association.
Conflict of interest statement
Figures







References
-
- Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, Casapia M, Santiago S, Gilbert P, Corey L. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant Adenovirus HIV vaccine. J Infect Dis. 2012;206(2):258–266. - PMC - PubMed
-
- NIH news statement. NIH discontinues immunizations in HIV vaccine study. 2013 Available from: http://www.niaid.nih.gov/news/newsreleases/2013/Pages/HVTN505 April2013.....
-
- García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong M, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. - PMC - PubMed
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JH, Jaffe HS, Martinez AI, Burns DN, Glidden DV. iPrEx Study Team, Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New Eng J Med. 2010;363:2587–2599. - PMC - PubMed
-
- Food and Drug Administration press release. FDA approves first medication to reduce HIV risk. 2012 Available from: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm311821.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

