Peginterferon alpha-2a versus peginterferon alpha-2b for chronic hepatitis C
- PMID: 24585451
- PMCID: PMC11040422
- DOI: 10.1002/14651858.CD005642.pub3
Peginterferon alpha-2a versus peginterferon alpha-2b for chronic hepatitis C
Abstract
Background: A combination of weekly pegylated interferon (peginterferon) alpha and daily ribavirin still represents standard treatment of chronic hepatitis C infection in the majority of patients. However, it is not established which of the two licensed peginterferon products, peginterferon alpha-2a or peginterferon alpha-2b, is the most effective and has a better safety profile.
Objectives: To systematically evaluate the benefits and harms of peginterferon alpha-2a versus peginterferon alpha-2b in head-to-head randomised clinical trials in patients with chronic hepatitis C.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and LILACS until October 2013. We also searched conference abstracts, journals, and grey literature.
Selection criteria: We included randomised clinical trials comparing peginterferon alpha-2a versus peginterferon alpha-2b given with or without co-intervention(s) (for example, ribavirin) for chronic hepatitis C. Quasi-randomised studies and observational studies as identified by the searches were also considered for assessment of harms. Our primary outcomes were all-cause mortality, liver-related morbidity, serious adverse events, adverse events leading to treatment discontinuation, other adverse events, and quality of life. The secondary outcome was sustained virological response in the blood serum.
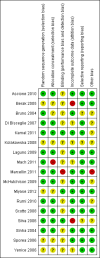
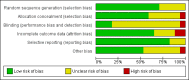
Data collection and analysis: Two authors independently used a standardised data collection form. We meta-analysed data with both the fixed-effect and the random-effects models. For each outcome we calculated the relative risk (RR) with 95% confidence interval (CI) based on intention-to-treat analysis. We used domains of the trials to assess the risk of systematic errors (bias) and trial sequential analyses to assess the risks of random errors (play of chance). Intervention effects on the outcomes were assessed according to GRADE.
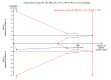
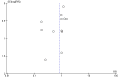
Main results: We included 17 randomised clinical trials which compared peginterferon alpha-2a plus ribavirin versus peginterferon alpha-2b plus ribavirin in 5847 patients. All trials had a high risk of bias. Very few trials reported data on very few patients for the patient-relevant outcomes all-cause mortality, liver-related morbidity, serious adverse events, and quality of life. Accordingly, we were unable to conduct meta-analyses on all-cause mortality, liver-related morbidity, and quality of life. Twelve trials reported on adverse events leading to discontinuation of treatment without clear evidence of a difference between the two peginterferons (197/2171 (9.1%) versus 311/3169 (9.9%); RR 0.84, 95% CI 0.57 to 1.22; I2 = 44%; low quality evidence). A trial sequential analysis showed that we could exclude a relative risk reduction of 20% or more on this outcome. Peginterferon alpha-2a significantly increased the number of patients who achieved a sustained virological response in the blood serum compared with peginterferon alpha-2b (1069/2099 (51%) versus 1327/3075 (43%); RR 1.12, 95% CI 1.06 to 1.18; I2= 0%, 12 trials; moderate quality evidence). Trial sequential analyses supported this result. Subgroup analyses based on risk of bias, viral genotype, and treatment history yielded similar results. Trial sequential analyses supported the results in patients with genotypes 1 and 4, but not in patients with genotypes 2 and 3.
Authors' conclusions: There is lack of evidence on patient-important outcomes and paucity of evidence on adverse events. Moderate quality evidence suggests that peginterferon alpha-2a is associated with a higher sustained virological response in serum than with peginterferon alpha-2b. This finding may be affected by the high risk of bias of the included studies . The clinical consequences of peginterferon alpha-2a versus peginterferon alpha-2b are unknown, and we cannot translate an effect on sustained virological response into comparable clinical effects because sustained virological response is still an unvalidated surrogate outcome for patient-important outcomes. The lack of evidence on patient-important outcomes and the paucity of evidence on adverse events means that we are unable to draw any conclusions about the effects of one peginterferon over the other.
Conflict of interest statement
Tahany Awad was an invited speaker for Roche and is now employed by AbbVie.
Figures




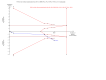
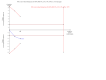



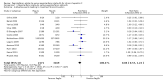






Update of
References
References to studies included in this review
Ascione 2010 {published data only}
-
- Ascione A, Luca M, Tartaglione MT, Lampasi F, Lanza AG, Picciotto FP, et al. Peginterferon alfa‐2a plus ribavirin Is more effective than peginterferon alfa‐2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology 2010;138(1):116‐22. - PubMed
-
- Ascione A, Luca M, Tartaglione MT, Lampasi F, Lanza AG, Picciotto FP, et al. Peginterferon alpha‐2a plus ribavirin versus peginterferon alpha‐2b plus ribavirin in naive patients with chronic hepatitis C virus infection: results of a prospective randomised trial. Abstracts of the 43rd Annual Meeting of the European Association for the Study of the Liver. Hepatology 2008;48(S2):370.
Berak 2005 {published data only}
-
- Berak H, Horban A, Wasilewski M, Stanczak JJ, Kolakowska‐Rzadzka A. Randomized, open label trial comparing efficacy and safety of pegylated interferon alfa 2A vs alfa 2B treatment of patients with chronic hepatitis C infected with non 2/3 genotypes‐12 week virological response analysis. Hepatology 2005;42(4 S1):684A.
Bruno 2004 {published data only}
-
- Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, et al. Viral dynamics and pharmacokinetics of peginterferon alpha‐2a and peginterferon alpha‐2b in naive patients with chronic hepatitis C: a randomized, controlled study. Antiviral Therapy 2004;9(4):491‐7. - PubMed
-
- Bruno R, Sacchi P, Maiocchi L, Zocchetti C, Ciappina V, Patruno S, et al. Area‐under‐the‐curve for peginterferon alpha‐2a and peginterferon alpha‐2b is not related to body weight in treatment‐naive patients with chronic hepatitis C. Antiviral Therapy 2005;10(2):201‐5. - PubMed
-
- Bruno R, Sacchi P, Scagnolari C, Torriani F, Maiocchi L, Patruno S, et al. Pharmacodynamics of peginterferon alpha‐2a and peginterferon alpha‐2b in interferon‐naïve patients with chronic hepatitis C: a randomized, controlled study. Alimentary Pharmacology & Therapeutics 2007;26(3):369‐76. - PubMed
Di Bisceglie 2007 {published data only}
-
- Bisceglie AM, Ghalib RH, Hamzeh FM, Rustgi VK. Early virologic response after peginterferon alpha‐2a plus ribavirin or peginterferon alpha‐2b plus ribavirin treatment in patients with chronic hepatitis C. Viral Hepatitis 2007;14(10):721‐9. - PubMed
-
- Bisceglie AM, Rustgi VK, Thuluvath P, Davis M, Ghalib R, Lyons MF, et al. Pharmacokinetics and pharmacodynamics of pegylated interferon alfa‐2A or alfa‐2B with ribavirin in treatment naive patients with genotype 1 chronic hepatitis C. Hepatology 2004;40(S4):734A.
Kamal 2011 {published data only}
-
- Kamal MS, Ahmed A, Mahmoud S, Nabegh L, Gohary I, Obadan I, et al. Enhanced efficacy of pegylated interferon alpha‐2a over pegylated interferon and ribavirin in chronic hepatitis C genotype 4A randomised trial and quality of life analysis. Liver International 2011;31:401‐11. - PubMed
Kolakowska 2008 {published data only}
-
- Kolakowska A, Berok H, Wasilewski M, Horbon A. Relevance between fibrosis and response to treatment with peginterferon alfa2a vs alfa2b with ribavirin in chronic hepatitis C genotype 3 patients. Randomized open label study. Hepatology 2008;48(4):1278.
Laguno 2009 {published data only}
-
- Laguno M, Cifuentes C, Murillas J, Veloso S, Larrousse M, Payeras A, et al. Randomized trial comparing pegylated interferon alpha‐2b versus pegylated interferon alpha‐2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology 2009;49(1):22‐31. - PubMed
Mach 2011 {published data only}
-
- Mach TH, Cieśla A, Warunek W, Janas‐Skulina U, Cibor D, Owczarek D. Efficacy of pegylated interferon alfa‑2aor alfa‑2b in combination with ribavirin in the treatment of chronic hepatitis caused by hepatitis C virus genotype 1b. Polskie Archiwum Medycyny Wewnetrznej 2011;121:434‐40. - PubMed
Marcellin 2011 {published data only}
McHutchison 2009 {published data only}
-
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa‐2b or alfa‐2a with ribavirin for treatment of hepatitis C infection. New England Journal of Medicine 2009;361(6):580‐93. - PubMed
Miyase 2012 {published data only (unpublished sought but not used)}
-
- Miyase S, Haraoka K, Ouchida Y, Morishita Y, Fujiyama S. Randomized trial of peginterferon a‐2a plus ribavirin versus peginterferon a‐2b plus ribavirin for chronic hepatitis C in Japanese patients. Journal of Gastroenterology 2012;47(9):1014‐21. - PubMed
-
- Miyase S, Morishita Y, Haraoka K, Ouchida Y, Fujiyama S. PEGIFNalpha‐2A plus ribavirin shows higher SVR rate compare with PEGIFNalpha‐2B plus ribavirin in elderly patients with chronic hepatitis C specially in IL‐28B major allele. Journal of Hepatology 2012;56(Suppl 2):S451.
Rumi 2010 {published data only}
-
- Rumi M, Aghemo A, Prati GM, D'Ambrosio R, Donato MF, Russo R, et al. Randomized study comparing peginterferon‐alfa2a plus ribavirin and peginterferon‐alfa2b plus ribavirin in naïve patients with chronic hepatitis C: final results of the Milan Safety Tolerability (MIST) study. Hepatology 2008;48(4):A212.
-
- Rumi M, Aghemo A, Prati GM, D'Ambrosio R, Donato MF, Russo R, et al. Randomized study of peginterferon‐∝2a plus ribavirin vs peginterferon‐∝2b plus ribavirin in chronic hepatitis C. Gastroenterology 2010;138(1):108‐15. - PubMed
Scotto 2008 {published data only}
-
- Scotto G, Fazio V, Fornabaio C, Tartaglia A, Tullio R, Saracino A, et al. Early and sustained virological response in non‐responders with chronic hepatitis C; a randomized open‐label study of pegylated interferon‐α‐2a versus pegylated interferon‐α‐2b. Drugs 2008;68(6):791‐801. - PubMed
-
- Scotto G, Fazio V, Fornabaio C, Tartaglia A, Tullio R, Saracino A, et al. Peg‐interferon alpha‐2a versus peg‐interferon alpha‐2b in non responders with HCV active chronic hepatitis: A pilot study. Interferon and Cytokine Research 2008;28:623–30. - PubMed
Silva 2006 {published data only}
-
- Silva M, Poo J, Wagner F, Jackson M, Cutler D, Grace M, et al. A randomised trial to compare the pharmacokinetic, pharmacodynamic, and antiviral effects of peginterferon alfa‐2b and peginterferon alfa‐2a in patients with chronic hepatitis C (COMPARE). Hepatology 2006;45(2):204‐13. - PubMed
-
- Silva M, Poo‐Ramirez JL, Wagner F, Jackson M, Laughlin M. Compare trial: Updated data. Hepatology 2008;49(2):288‐9. - PubMed
-
- Silva M, Poo‐Ramirez JL, Wagner F, Jackson M, Laughlin M. Comparison of peginterferon‐alpha2a and peginterferon‐alpha2B pharmacokinetics and pharmacodynamic in compare, a randomized, prospective, blinded trial. Hepatology 2004;40(4 S1):192A.
Sinha 2004 {published data only}
-
- Sinha S, Gulur P, Patel V, Hage‐Nassar G, Tenner S. A randomized prospective clinical trial comparing pegylated interferon alpha 2a/ribavirin versus pegylated interferon alpha 2b/ribavirin in the treatment of chronic hepatitis C. American Journal of Gastroenterology 2004;99(10):237.
Sporea 2006 {published data only}
-
- Sporea I, Danila M, Sirli R, Popescu A, Laza A, Baditoiu L. Comparative study concerning the efficacy of Peg‐IFN alpha‐2a versus Peg‐IFN alpha‐2b on the early virological response (EVR) in patients with chronic viral C hepatitis. Journal of Gastrointestinal and Liver Diseases 2006; Vol. 15, issue 2:125‐30. - PubMed
Yenice 2006 {published data only}
-
- Yenice N, Mehtap O, Gumrah M, Arican N. The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turkish Journal of Gastroenterology 2006;17(2):94‐8. - PubMed
References to studies excluded from this review
Andrade 2006 {published data only}
-
- Andrade RJ, González FJ, Vázquez L, Cilvetti A, Camargo R, García‐Cortés M, et al. Vascular ophthalmological side effects associated with antiviral therapy for chronic hepatitis C are related to vascular endothelial growth factor levels. Antiviral Therapy 2006;11(4):491‐8. - PubMed
Barros 2010a {published data only}
Barros 2010b {published data only}
Bruchfeld 2006 {published data only}
-
- Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. Journal of Viral Hepatology 2006;13(5):316‐21. - PubMed
Cozzolongo 2006 {published data only}
-
- Cozzolongo R, Sartorie M, Lanzilotta E, Tonello N, Manghisi OG. Comparison between the two peginterferon's a in the treatment of chronic hepatitis C. Journal of Hepatology 2006;44(S2):209.
Craxi 2008 {published data only}
-
- Craxi A, Piccinino F, Alberti A. Predictors of SVR in naïve HCV G1 patients in real life practice: the probe. Journal of Hepatology 2008;2(48):S291.
El Raziky 2013 {published data only}
Escudero 2008 {published data only}
-
- Escudero A, Rodríguez F, Serra MA, Olmo J, Montes F, Rodrigo JM. Pegylated interferon‐2a plus ribavirin compared with pegylated interferon‐2b plus ribavirin for initial treatment of chronic hepatitis C virus: Prospective, non‐randomized study. Journal of Gastroenterology and Hepatology 2008;23(6):861‐6. - PubMed
Espinosa 2007 {published data only}
-
- Espinosa M, Arenas MD, Aumente MD, Barril G, Buades JM, Aviles B, et al. Anemia associated with pegylated interferon‐alpha 2a and alpha 2b therapy in haemodialysis patients. Clinical Nephrology 2007;67:366‐73. - PubMed
Hofmann 2006 {published data only}
-
- Hofmann WP, Bock H, Weber C, Tacke W, Pfaff R, Kihn R, et al. Effectiveness of antiviral therapy in patients with chronic hepatitis C treated by private practice gastroenterologists. Zeitschrift für Gastroenterologie 2006;44(1):25‐31. - PubMed
Lee 2010 {published data only}
-
- Lee S, Kim IH, Kim SH, Kim SW, Lee SO, Lee ST. Efficacy and tolerability of pegylated interferon‐alpha 2a plus ribavirin versus pegylated interferon‐ alpha 2b plus ribavirin in treatment‐naive chronic hepatitis C patients. Intervirology 2010;53:146‐53. - PubMed
Rumi 2012 {published data only}
-
- Rumi M, Aghemo A, Prati GM. Comparative trials of peginterferon alpha 2a and peginterferon alpha 2b for chronic hepatitis C. Journal of Viral Hepatitis 2012;19:37‐41. - PubMed
Villa 2012 {published data only}
-
- Villa E, Camma C, Leo A, Karampatou A, Enea M, Gitto S, et al. Peginterferon‐A 2B plus ribavirin is more effective than peginterferon‐A 2A plus ribavirin in menopausal women with chronic hepatitis C. Journal of Viral Hepatitis 2012;19:640–9. - PubMed
Witthoeft 2008 {published data only}
-
- Witthoeft T, Hueppe D, John C, Goelz J, Meyer U, Heyne R, et al. Efficacy and safety of peginterferon alfa‐2a or ‐2b plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Germany: The practice study. Abstracts of the 43rd Annual Meeting of the European Association for the Study of the Liver. Hepatology 2008;48(S2):315.
Additional references
Adeel 2009
-
- Adeel AB, Xiao QW, Charity GM. Effect of hepatitis C virus and its treatment on survival. Hepatology 2009;50(2):387‐92. - PubMed
Alavian 2010
Als‐Nielsen 2003
-
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: A reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. - PubMed
Bailon 2001
-
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung W‐J, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis. Bioconjugate Chemistry 2001;12(2):195‐202. - PubMed
Bangalore 2008
-
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli F. Perioperative β blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet 2008;372(9654):1962‐76. - PubMed
Barros 2010c
-
- Barros FMR, Cheinquer H, Borges LG, Santos E. Is there any difference between the effects of therapy with peginterferon‐alpha‐2a versus standard‐dose peginterferon‐alpha‐2b? A meta‐analysis comparing both treatments plus ribavirin in genotype 1/4 chronic hepatitis C virus (HCV) infection patients. Value in Health 2010;13:A368.
Benvegnu 2001
-
- Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma development in hepatitis B virus and hepatitis C virus‐related cirrhosis. Antiviral Research 2001;52:199‐207. - PubMed
Brok 2005
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment for random error risk in conclusive Cochrane neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Chan 2013
Cheinquer 2010
-
- Cheinquer H, Barros FMR, Borges LG, Santos E, Buschinelli CT. Systematic review and meta‐analysis of published randomised controlled trials comparing the efficacy of peginterferon‐alpha‐2a versus peginterferon alpha‐2b both plus ribavirin in chronic hepatitis C patients. Value in Health 2010;13:A69.
Coppola 2011
-
- Coppola N, Pisaturo M, Sagnelli C, Tonziello G, Sagnelli E, Angelillo IF. Efficacy and tolerability of peginterferon α‐2a and α‐2b in patients with chronic hepatitis C by genotype 1: A meta‐analysis. Digestive and Liver Diseases 2011;43(Suppl):S94.
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 27 May 2013).
Davis 1999
-
- Davis GL. Hepatitis C virus genotypes and quasi species. American Journal of Medicine 1999;107(6B):21S‐6S. - PubMed
DeMets 1987
-
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Di Biscceglie 2011
Druyits 2012
EASL 2012
-
- European Association for the Study of the Liver. European Association for the Study of the Liver, EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. Journal of Hepatology 2011;55(2):245‐64. - PubMed
Egger 1997
Fattovich 2002
-
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, European Concerted Action on Viral Hepatitis (EUROHEP). Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. American Journal of Gastroenterology 2002;97(11):288‐95. - PubMed
Fernandez‐ Rodriguez 2010
-
- Fernández‐Rodríguez CM, Alonso S, Martinez SM, Forns X, Sanchez‐Tapias JM, Rincón D. Peginterferon plus ribavirin and sustained virological response in HCV‐related cirrhosis: outcomes and factors predicting response. American Journal of Gastroenterology 2010;105:2164‐72. - PubMed
Flori 2013
-
- Flori N, Funakoshi N, Duny Y, Valats JC, Bismuth M, Christophorou D, et al. Pegylated interferon‐a2a and ribavirin versus pegylated interferon‐a2b and ribavirin in chronic hepatitis C. A meta‐analysis. Drugs 2013;73:263‐77. - PubMed
Foster 2004
-
- Foster GR. Pegylated interferons: chemical and clinical differences. Alimentary Pharmacology & Therapeutics 2004;20(8):825‐30. - PubMed
Fried 2002
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Human Services 2002;347:975–82. - PubMed
Ghany 2009
Ghany 2011
Glue 2000
-
- Glue P, Fang JWS, Rouzier‐Panis R, Raffanel C, Sabo R, Gupta SK, et al. Pegylated interferon‐alpha 2b: Pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clinical Pharmacology and Therapeutics 2000;68(5):556‐67. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 12. Art. No.: LIVER.
Gurusamy 2014
-
- Gurusamy KS, Wilson E, Koretz RL, Allen VB, Davidson BR, Burroughs AK, et al. Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?. PLoS ONE 2014;8(12):e83313. - PMC - PubMed
Guyatt 2008
Hadziyannis 2004
-
- Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon alfa‐2a (40 kilodaltons) and ribavirin combination therapy in chronic hepatitis C: randomized study of the effect of treatment duration and ribavirin dose. Annals of Internal Medicine 2004;140:346–55. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirofumi 2009
Hodgson 2003
-
- Hodgson HJF. Viral hepatitis ‐ clinical aspects. In: Warrell DA, Cox TM, Firth JD, Benz EJ Jr editor(s). Oxford Textbook of Medicine. 4th Edition. Oxford/ New York: Oxford University Press, 2003.
Hollis 1999
Hopewell 2009
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Ioannidis 2005
-
- Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005;294(2):218‐28. - PubMed
Ioannidis 2005a
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koretz 2013
Lacchetti 2002
-
- Lacchetti C, Guyatt G. Therapy and validity: surprising results of randomized controlled trials. In: Guyatt G, Rennie D editor(s). Users' Guides to the Medical Literature: A Manual for Evidence‐Based Clinical Practice. Chicago: AMA Press, 2002:247‐65.
Lau 1995
-
- Lau J, Schmid C, Chalmers T. Cumulative meta‐analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45‐57. - PubMed
Lee 2008
Lexchin 2003
Lundh 2012
Manns 2001
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358(9286):958‐65. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Moher 2012
-
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10:28‐55. - PubMed
Morgan 2010
Myers 2002
OPTN 2008
-
- United Network for Organ Sharing. Chapter VI ; Liver and Intestine Transplantation in the United States, 1998‐2007. optn.transplant.hrsa.gov/ar2008/chapter_iv_AR_cd.htm?cp=5 (accessed 27 May 2013).
Pasut 2011
-
- Pasut G, Veronese FM. State of the art in PEGylation: The great versatility achieved after forty years of research. Journal of Controlled Release 2011;161:461‐72. - PubMed
Penin 2004
Rambaldi 2008
-
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Glucocorticosteroids for alcoholic hepatitis – a Cochrane Hepato‐Biliary Group Systematic Review with meta‐analyses and trial sequential analyses of randomised clinical trials. Alimentary Pharmacology & Therapeutics 2008;27(12):1167‐78. - PubMed
Reddy 2001
-
- Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40‐kd) interferon alpha‐2a compared with interferon alpha‐2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001;33(2):433–8. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Romero‐Gomez 2012
-
- Romero‐Gomez M, Planas R, Sola R, Garcıa‐Samaniego J, Diago M, Crespo J, et al. Peginterferon alpha‐2A achieves higher early virological responses (RVR and CEVR) than peginterferon alpha‐2B in chronic Hepatitis C: Meta‐analysis of randomized clinical trials (RCT). Journal of Hepatology 2012;56:S456.
Rosenberg 2001
-
- Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. Journal of Molecular Biology 2001;313:451‐64. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. - PubMed
Savovic 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157:429‐38. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Seef 2009
Seeff 2002
Singal 2011
-
- Singal AK, Jampana SC, Anand BS. Peginterferon alfa‐2a is superior to peginterferon alfa‐2b in the treatment of naïve patients with hepatitis C virus infection: meta‐analysis of randomized controlled trials. Digestive Diseases and Sciences 2011;56:2221‐26. - PubMed
Thorlund 2009
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 April 2013):1‐115.
Trikalinos 2004
-
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57(11):1124‐30. - PubMed
van Regenmorte 2000
-
- Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, et al. Virus Taxonomy: The Classification and Nomenclature of Viruses. The Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 2000:599‐621.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
WHO 1999
-
- World Health Organization. Global surveillance and control of hepatitis C. Journal of Viral Hepatitis 1999;6(1):35‐47. - PubMed
WHO 2009
-
- WHO. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed 27 May 2013).
Wood 2008
Yang 2013
Zhao 2010
-
- Zhao S, Liu E, Chen P, Cheng D, Lu S, Yu Q, et al. A comparison of peginterferon α‐2a and α‐2b for treatment‐naive patients with chronic hepatitis C virus: A meta‐analysis of randomised trials. Clinical Therapeutics 2010;32:1565‐77. - PubMed
References to other published versions of this review
Awad 2009
Awad 2010
-
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51(4):1176‐84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

