Formal education of patients about to undergo laparoscopic cholecystectomy
- PMID: 24585482
- PMCID: PMC6823253
- DOI: 10.1002/14651858.CD009933.pub2
Formal education of patients about to undergo laparoscopic cholecystectomy
Abstract
Background: Generally, before being operated on, patients will be given informal information by the healthcare providers involved in the care of the patients (doctors, nurses, ward clerks, or healthcare assistants). This information can also be provided formally in different formats including written information, formal lectures, or audio-visual recorded information.
Objectives: To compare the benefits and harms of formal preoperative patient education for patients undergoing laparoscopic cholecystectomy.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2013), MEDLINE, EMBASE, and Science Citation Index Expanded to March 2013.
Selection criteria: We included only randomised clinical trials irrespective of language and publication status.
Data collection and analysis: Two review authors independently extracted the data. We planned to calculate the risk ratio with 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) with 95% CI for continuous outcomes based on intention-to-treat analyses when data were available.
Main results: A total of 431 participants undergoing elective laparoscopic cholecystectomy were randomised to formal patient education (215 participants) versus standard care (216 participants) in four trials. The patient education included verbal education, multimedia DVD programme, computer-based multimedia programme, and Power Point presentation in the four trials. All the trials were of high risk of bias. One trial including 212 patients reported mortality. There was no mortality in either group in this trial. None of the trials reported surgery-related morbidity, quality of life, proportion of patients discharged as day-procedure laparoscopic cholecystectomy, the length of hospital stay, return to work, or the number of unplanned visits to the doctor. There were insufficient details to calculate the mean difference and 95% CI for the difference in pain scores at 9 to 24 hours (1 trial; 93 patients); and we did not identify clear evidence of an effect on patient knowledge (3 trials; 338 participants; SMD 0.19; 95% CI -0.02 to 0.41; very low quality evidence), patient satisfaction (2 trials; 305 patients; SMD 0.48; 95% CI -0.42 to 1.37; very low quality evidence), or patient anxiety (1 trial; 76 participants; SMD -0.37; 95% CI -0.82 to 0.09; very low quality evidence) between the two groups.A total of 173 participants undergoing elective laparoscopic cholecystectomy were randomised to electronic consent with repeat-back (patients repeating back the information provided) (92 participants) versus electronic consent without repeat-back (81 participants) in one trial of high risk of bias. The only outcome reported in this trial was patient knowledge. The effect on patient knowledge between the patient education with repeat-back versus patient education without repeat-back groups was imprecise and based on 1 trial of 173 participants; SMD 0.07; 95% CI -0.22 to 0.37; very low quality evidence).
Authors' conclusions: Due to the very low quality of the current evidence, the effects of formal patient education provided in addition to the standard information provided by doctors to patients compared with standard care remain uncertain. Further well-designed randomised clinical trials of low risk of bias are necessary.
Conflict of interest statement
None known.
Figures
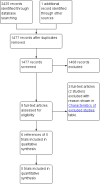
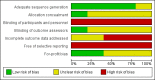







Update of
References
References to studies included in this review
Blay 2005 {published data only}
-
- Blay N, Donoghue J. The effect of pre‐admission education on domiciliary recovery following laparoscopic cholecystectomy. Australian Journal of Advanced Nursing 2005;22(4):14‐9. - PubMed
Bollschweiler 2008 {published data only}
-
- Bollschweiler E, Apitsch J, Obliers R, Koerfer A, Monig SP, Metzger R, et al. Improving informed consent of surgical patients using a multimedia‐based program? Results of a prospective randomized multicenter study of patients before cholecystectomy. Annals of Surgery 2008;248(2):205‐11. - PubMed
Clark 2011 {published data only}
-
- Clark S, Mangram A, Ernest D, Lebron R, Peralta L. The informed consent: a study of the efficacy of informed consents and the associated role of language barriers. Journal of Surgical Education 2011;68(2):143‐7. - PubMed
Fink 2010 {published data only}
-
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Annals of Surgery 2010;252(1):27‐36. - PubMed
-
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Predictors of comprehension during surgical informed consent. Journal of the American College of Surgeons 2010;210(6):919‐26. - PubMed
Wilhelm 2009 {published data only}
-
- Wilhelm D, Gillen S, Wirnhier H, Kranzfelder M, Schneider A, Schmidt A, et al. Extended preoperative patient education using a multimedia DVD‐impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial. Langenbeck's Archives of Surgery 2009;394(2):227‐33. - PubMed
References to studies excluded from this review
Broadbent 2012 {published data only}
-
- Broadbent E, Kahokehr A, Booth RJ, Thomas J, Windsor JA, Buchanan CM, et al. A brief relaxation intervention reduces stress and improves surgical wound healing response: a randomised trial. Brain, Behavior and Immunity 2012;26(2):212‐7. - PubMed
Stergiopoulou 2007 {published data only}
-
- Stergiopoulou A, Birbas K, Katostara I, Mantas J. The effect of interactive multimedia on preoperative knowledge and postoperative recovery of patients undergoing laparoscopic cholecystectomy. Methods of Information in Medicine 2007;46(4):406‐9. - PubMed
-
- Stergiopoulou A, Birbas K, Katostaras T, Diomidous M, Mantas J. The effect of a multimedia health educational program on the postoperative recovery of patients undergoing laparoscopic cholecystectomy. Studies in Health Technology and Informatics 2006;124:920‐5. - PubMed
Additional references
Attili 1995
-
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995;21(3):655‐60. - PubMed
Ballal 2009
-
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. - PubMed
Bates 1992
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Cassady 1999
-
- Cassady JF Jr, Wysocki TT, Miller KM, Cancel DD, Izenberg N. Use of a preanesthetic video for facilitation of parental education and anxiolysis before pediatric ambulatory surgery. Anesthesia and Analgesia 1999;88(2):246‐50. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 6 January 2014).
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dolan 2009
-
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. - PubMed
Egger 1997
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 8. Art. No.: LIVER.
GREPCO 1984
-
- GREPCO. Prevalence of gallstone disease in an Italian adult female population. Rome group for the epidemiology and prevention of cholelithiasis (GREPCO). American Journal of Epidemiology 1984;119(5):796‐805. - PubMed
GREPCO 1988
-
- GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part i. Prevalence data in men. The Rome group for epidemiology and prevention of cholelithiasis (GREPCO). Hepatology 1988;8(4):904‐6. - PubMed
Gurusamy 2008a
Gurusamy 2008b
-
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. - PubMed
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. - PubMed
Halldestam 2004
-
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. - PubMed
HES 2011
-
- HESonline. Hospital Episode Statistics. Main procedures and interventions: 3 character, 2011. www.hscic.gov.uk/hes (accessed 6 January 2014).
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
McDonald 2004
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NIH 1992
-
- National Institutes of Health. NIH consensus statement on gallstones and laparoscopic cholecystectomy, 1992. consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm (accessed 6 January 2014).
O'Connor 2009
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Sinha 2013
-
- Sinha S, Hofman D, Stoker DL, Friend PJ, Poloniecki JD, Thompson MM, et al. Epidemiological study of provision of cholecystectomy in England from 2000 to 2009: retrospective analysis of Hospital Episode Statistics. Surgical Endoscopy 2013;27(1):162‐75. - PubMed
SPIRIT 2013
SPIRIT 2013a
Strasberg 1993
-
- Strasberg SM, Clavien PA. Overview of therapeutic modalities for the treatment of gallstone diseases. American Journal of Surgery 1993;165(4):420‐6. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 6 January 2014).
Todd 1996
-
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

