Phase II study of gefitinib in patients with advanced salivary gland cancers
- PMID: 24585506
- PMCID: PMC5669372
- DOI: 10.1002/hed.23647
Phase II study of gefitinib in patients with advanced salivary gland cancers
Abstract
Background: The purpose of this study was to determine the antitumor activity of the epidermal growth factor receptor (EGFR) inhibitor gefitinib in patients with recurrent/metastatic salivary gland cancer.
Methods: We conducted a phase II study in adenoid cystic carcinoma (ACC) and non-ACC. Gefitinib was administered 250 mg orally daily. The primary endpoint was tumor response. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and disease control rates. EGFR and human epidermal growth factor receptor 2 (HER2) expression were evaluated and correlated with outcomes.
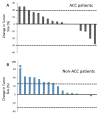
Results: Thirty-seven patients were enrolled in this study, and 36 were evaluable (18 with ACC and 18 with non-ACC). No responses were observed. Median PFS was 4.3 months and 2.1 months, and median OS was 25.9 months and 16 months for patients with ACC and non-ACC, respectively. The disease control rate at 8 weeks was higher in patients with ACC. No unexpected toxicities occurred. EGFR and HER2 overexpression did not correlate with outcomes.
Conclusion: We did not observe significant clinical activity of gefitinib in advanced salivary gland cancer. NCT00509002.
Keywords: adenoid cystic carcinoma; gefitinib; non-adenoid cystic carcinoma; response to therapy; salivary gland cancer.
© 2015 Wiley Periodicals, Inc.
Figures
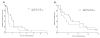
Similar articles
-
Phase II trial with axitinib in recurrent and/or metastatic salivary gland cancers of the upper aerodigestive tract.Head Neck. 2019 Oct;41(10):3670-3676. doi: 10.1002/hed.25891. Epub 2019 Jul 29. Head Neck. 2019. PMID: 31355973 Clinical Trial.
-
A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact.Eur J Cancer. 2016 Dec;69:158-165. doi: 10.1016/j.ejca.2016.09.022. Epub 2016 Nov 5. Eur J Cancer. 2016. PMID: 27821319 Clinical Trial.
-
Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands.J Clin Oncol. 2007 Sep 1;25(25):3978-84. doi: 10.1200/JCO.2007.11.8612. J Clin Oncol. 2007. PMID: 17761983 Clinical Trial.
-
Systemic treatments in recurrent or metastatic salivary gland cancer: a systematic review.ESMO Open. 2024 Oct;9(10):103722. doi: 10.1016/j.esmoop.2024.103722. Epub 2024 Oct 4. ESMO Open. 2024. PMID: 39368417 Free PMC article.
-
Systemic therapy in the palliative management of advanced salivary gland cancers.J Clin Oncol. 2006 Jun 10;24(17):2673-8. doi: 10.1200/JCO.2005.05.3025. J Clin Oncol. 2006. PMID: 16763282 Review.
Cited by
-
Genomic landscape of salivary gland tumors.Oncotarget. 2015 Sep 22;6(28):25631-45. doi: 10.18632/oncotarget.4554. Oncotarget. 2015. PMID: 26247885 Free PMC article.
-
Particle Beam Radiation Therapy for Adenoid Cystic Carcinoma of the Nasal Cavity and Paranasal Sinuses.Front Oncol. 2020 Sep 30;10:572493. doi: 10.3389/fonc.2020.572493. eCollection 2020. Front Oncol. 2020. PMID: 33102230 Free PMC article.
-
Retrospective Study of Cisplatin/Carboplatin, 5-Fluorouracil Plus Cetuximab (EXTREME) for Advanced-stage Salivary Gland Cancer.In Vivo. 2022 Mar-Apr;36(2):979-984. doi: 10.21873/invivo.12790. In Vivo. 2022. PMID: 35241559 Free PMC article.
-
Salivary glands adenoid cystic carcinoma: a molecular profile update and potential implications.Front Oncol. 2023 Jul 5;13:1191218. doi: 10.3389/fonc.2023.1191218. eCollection 2023. Front Oncol. 2023. PMID: 37476370 Free PMC article. Review.
-
Mutation analysis of the EGFR pathway genes, EGFR, RAS, PIK3CA, BRAF, and AKT1, in salivary gland adenoid cystic carcinoma.Oncotarget. 2018 Mar 30;9(24):17043-17055. doi: 10.18632/oncotarget.24818. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682203 Free PMC article.
References
-
- Adelstein DJ, Rodriguez CP. What is new in the management of salivary gland cancers? Curr Opin Oncol. 2011;23:249–53. - PubMed
-
- Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229–40. - PubMed
-
- NCCN. National Comprehensive Cancer Network v2.2012 ed. 2012. [Accessed 11/6/212]. Head and Neck Cancers: Salivary Gland Cancers.
-
- Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002;24:632–6. - PubMed
-
- Choi S, Sano D, Cheung M, Zhao M, Jasser SA, Ryan AJ, Mao L, Chen WT, El-Naggar AK, Myers JN. Vandetanib inhibits growth of adenoid cystic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2008;14:5081–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

