Peginterferon plus ribavirin versus interferon plus ribavirin for chronic hepatitis C
- PMID: 24585509
- PMCID: PMC11053364
- DOI: 10.1002/14651858.CD005441.pub3
Peginterferon plus ribavirin versus interferon plus ribavirin for chronic hepatitis C
Abstract
Background: Pegylated interferon (peginterferon) plus ribavirin is the recommended treatment for patients with chronic hepatitis C, but systematic assessment of the effect of this treatment compared with interferon plus ribavirin is needed.
Objectives: To systematically evaluate the benefits and harms of peginterferon plus ribavirin versus interferon plus ribavirin for patients with chronic hepatitis C.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index-Expanded, and LILACS. We also searched conference abstracts, journals, and grey literature. The last searches were conducted in September 2013.
Selection criteria: We included randomised clinical trials comparing peginterferon plus ribavirin versus interferon plus ribavirin with or without co-intervention(s) (e.g., other antiviral drugs) for chronic hepatitis C. Quasi-randomised and observational studies retrieved through the searches for randomised clinical trials were also considered for reports of harms. Our primary outcomes were liver-related morbidity, all-cause mortality, serious adverse events, adverse events leading to treatment discontinuation, other adverse events, and quality of life. Our secondary outcome was sustained virological response in serum, that is, undetectable hepatitis C virus RNA in serum by sensitive tests six months after the end of treatment.
Data collection and analysis: Two review authors independently used a standardised data collection form. We meta-analysed data with both fixed-effect and random-effects models. For each outcome, we calculated the odds ratio (OR) (for liver-related morbidity or all-cause mortality) or the risk ratio (RR) along with 95% confidence interval (CI) based on intention-to-treat analysis. We used domains of the trials to assess the risk of systematic errors (bias) and trial sequential analyses to assess the risk of random errors (play of chance).For each outcome, we calculated the RR with 95% CI based on intention-to-treat analysis. Effects of interventions on outcomes were assessed according to GRADE.
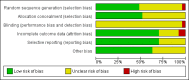
Main results: We included 27 randomised trials with 5938 participants. All trials had high risk of bias. We considered that the risk of bias did not impact on the quality of evidence for liver-related mortality and adverse event outcomes, but it did for virological response. All trials compared peginterferon alpha-2a or peginterferon alpha-2b plus ribavirin versus interferon plus ribavirin for participants with chronic hepatitis C. Three trials administered co-interventions (amantadine hydrochloride 200 mg daily to both intervention groups), and 24 trials were conducted without co-interventions. The effect observed between the two intervention groups regarding liver-related morbidity plus all-cause mortality (5/907 (0.55%) versus 4/882 (0.45%) was imprecise: OR 1.14 ( 95% CI 0.38 to 3.42; five trials; low quality of evidence), as was the risk of adverse events leading to treatment discontinuation (332/2692 (12.3%) versus 409/2176 (18.8%); RR 0.86, 95% CI 0.68 to 1.09; 15 trials; low quality of evidence) or regarding adverse events leading to treatment discontinuation (332/2692 (12.3%) versus 409/2176 (18.8%); RR 0.86, 95% CI 0.66 to 1.12; 17 trials; low quality of evidence). However, peginterferon plus ribavirin versus interferon plus ribavirin significantly increased the risk of neutropenia (332/2202 (15.1%) versus 117/1653 (7.1%); RR 2.15, 95% CI 1.76 to 2.61; 13 trials), thrombocytopenia (65/1113 (5.8%) versus 23/1082 (2.1%); RR 2.63, 95% CI 1.68 to 4.11; 10 trials), arthralgia (517/1740 (29.7%) versus 282/1194 (23.6%); RR 1.19, 95% CI 1.05 to 1.35; four trials), injection site reaction (627/1168 (53.7%) versus 186/649 (28.7%); RR 1.71, 95% CI 1.50 to 1.93; four trials), and nausea (606/1784 (34.0%) versus 354/1239 (28.6%); RR 1.13, 95% CI 1.01 to 1.26; four trials). The most frequent adverse event was fatigue, which occurred in 57% of participants (2024/3608). No significant difference was noted between peginterferon plus ribavirin versus interferon plus ribavirin in terms of fatigue (1177/2062 (57.1%) versus 847/1546 (54.8%); RR 1.01, 95% CI 0.96 to 1.07; 12 trials). No significant differences were reported between the two treatment groups regarding anaemia, headache, rigours, myalgia, pyrexia, weight loss, asthenia, depression, insomnia, irritability, alopecia, pruritus, skin rash, thyroid malfunction, decreased appetite, or diarrhoea. We were unable to identify any data on quality of life. Peginterferon plus ribavirin versus interferon plus ribavirin seemed to significantly increase the number of participants achieving sustained virological response (1673/3300 participants (50.7%) versus 1081/2804 patients (36.7%); RR 1.39, 95% CI 1.25 to 1.56; I2 = 64%; 27 trials; very low quality of evidence). However, the risk of bias in the 13/27 (48.1%) trials reporting on this outcome was high and was considered only 'lower' in the remainder. Because the conventional meta-analysis did not reach its required information size (n = 14,486 participants), we used trial sequential analysis to control for risks of random errors. Again, in this analysis, the estimated effect was statistically significant in favour of peginterferon. Subgroup analyses according to risk of bias, viral genotype, baseline viral load, past treatment history, and type of intervention yielded similarly significant results favouring peginterferon over interferon on the outcome of sustained virological response.
Authors' conclusions: Peginterferon plus ribavirin versus interferon plus ribavirin seems to significantly increase the proportion of patients with sustained virological response, as well as the risk of certain adverse events. However, we have insufficient evidence to recommend or reject peginterferon plus ribavirin for liver-related morbidity plus all-cause mortality compared with interferon plus ribavirin. The clinical consequences of achieved sustained virological response are unknown, as sustained virological response is still an unvalidated surrogate outcome. We found no evidence of the potential benefits on quality of life in patients with achieved sustained virological response. Further high-quality research is likely to have an important impact on our confidence in the estimate of patient-relevant outcomes and is likely to change our estimates.There is very low quality evidence that peginterferon plus ribavirin increases the proportion of patients with sustained virological response in comparison with interferon plus ribavirin. There is evidence that it also increases the risk of certain adverse events.
Conflict of interest statement
Tahany Awad acted as an invited speaker for Roche and is now employed by AbbVie.
Figures







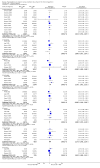





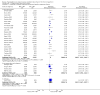



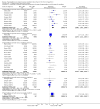
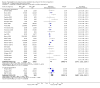
Update of
- doi: 10.1002/14651858.CD005441.pub2
References
References to studies included in this review
Al‐Faleh 2004 {published data only}
-
- Alfaleh FZ, Hadad Q, Khuroo MS, Aljumah A, Algamedi A, Alashgar H, et al. Peginterferon alpha‐2b plus ribavirin compared with interferon alpha‐2b plus ribavirin for initial treatment of chronic hepatitis C in Saudi patients commonly infected with genotype 4. Liver International 2004;24:568‐74. - PubMed
Bruno 2004 {published data only}
-
- Bruno S, Camma C, Marco V, Rumi M, Vinci M, Camozzi M, et al. Peginterferon alfa‐2b plus ribavirin for naive patients with genotype 1 chronic hepatitis C: a randomized controlled trial. Journal of Hepatology 2004;41:474‐81. - PubMed
-
- Bruno S, Camma C, Marco V, Rumi MG, Vinci M, Camozzi M, et al. Chronic hepatitis C (CH‐C) genotype 1: an independent, multicenter RCT comparing PEG‐IFN alfa‐2b 12 kD plus ribavirin (RBV) and IFN alfa‐2b plus RBV in naive patients. Journal of Hepatology 2003;38(Suppl 2):131‐2.
Cariti 2002 {published data only}
-
- Cariti G, Andreoni M, Calleri G, Manca A, Rosina F, Sartori M, et al. PEG‐INF alpha‐2A (40KD) plus ribavirin (RBV) vs IFN alpha‐2A (ROFERON‐A) in naive patients with chronic hepatitis C (CHC) and genotype 1 [AASLD abstract]. Hepatology 2002;36(4 Pt 2):595A.
Derbala 2005 {published data only}
Derbala 2006 {published data only}
Dollinger 2005 {published data only}
-
- Dollinger MM, Dridi Y, Lesske J, Behl S, Fleig WE. Efficacy of daily consensus interferon and ribavirin compared to peg‐interferon alpha‐2B and interferon in non‐responders with chronic hepatitis C [AASLD abstract]. Hepatology 2005;42(4 Suppl 1):691‐2.
Esmat 2003 {published data only}
-
- Esmat GH, Abouzied A, Abdel‐Hamid M, Mohamed MK, Zalata K, Raziky MS, et al. Results of a randomized clinical trial of genotype‐4 infected subjects when treated with standard or pegylated interferon alfa‐2b in combination with ribavirin [AASLD abstract]. Hepatology 2003;38(4 Suppl 1):314A‐315A.
Fargion 2004 {published data only}
-
- Fargion S, Borzio M, Cargnel A. End of treatment and sustained response to peginterferon alfa‐2a (40kD) (Pegasys) plus ribavirin (RBV) (Copegus) and amantadine (AMA), and to induction therapy with interferon (IFN) alfa‐2a (Roferon‐A) plus RBV and AMA in IFN/RBV nonresponders with chronic hepatitis C (CHC) [AASLD abstract]. Hepatology 2003;38(4 Suppl 1):733A.
-
- Fargion S, Borzio M, Cargnel A. Peginterferon alfa‐2a (40kD) (Pegasys) plus ribavirin (RBV) and amantadine (AMA) vs induction therapy with interferon alfa‐2a (Roferon‐A) plus RBV and AMA in IFN/RBV nonresponder patients with chronic hepatitis C (CHC). Journal of Hepatology 2003;38(Suppl 2):139‐40.
-
- Fargion S, Borzio M, Cargnel A. Peginterferon alfa‐2a (40kD) (Pegasys) plus ribavirin (RBV) and amantadine (AMA) vs induction therapy with interferon alfa‐2a (Roferon‐A) plus RBV and AMA in IFN/RBV nonresponder patients with chronic hepatitis C (CHC) [AASLD abstract]. Hepatology 2002;36(4 Pt 2):572A.
-
- Fargion S, Borzio M, Maraschi A, Cargnel A. Sustained virological response (SVR) to peginterferon alfa‐2a (40 kD) (Pegasys) plus ribavirin (RBV) and amantadine (AMA) vs induction therapy with interferon alfa‐2a (Roferon‐A) plus RBV and AMA in IFN/RBV non responders (NR) with chronic hepatitis C (CHC). Journal of Hepatology 2004;40(Suppl 1):140.
Fried 2002 {published data only}
-
- Bosques‐Padilla F, Trejo‐Estrada R, Campollo‐Rivas O, Cortez‐Hernandez C, Dehesa‐Violante M, Maldonado‐Garza H, et al. Peginterferon alfa‐2a plus ribavirin for treating chronic hepatitis C virus infection: analysis of Mexican patients included in a multicenter international clinical trial. Annals of Hepatology 2003;2(3):135‐9. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 2002;347(13):975‐82. - PubMed
-
- Hassanein T, Cooksley G, Sulkowski M, Smith C, Marinos G, Lai M‐Y, et al. The impact of peginterferon alfa‐2a plus ribavirin combination therapy on health‐related quality of life in chronic hepatitis C. Journal of Hepatology 2004;40(4):675‐81. - PubMed
-
- Hassanein TI, Cooksley G, Sulkowski M, Smith C, Marinos G, Lai M‐Y, et al. Treatment with 40 kDa peginterferon alfa‐2a (Pegasys) in combination with ribavirin significantly enhances quality of life compared with interferon alfa‐2b plus ribavirin [AASLD abstract]. Hepatology 2001;34(4 Pt 2):243A.
-
- Hassanein TI, Cooksley WGE, Sulkowski M, Smith C, Marinos G, Lai M‐Y, et al. QOL benefits observed as early as week 2 with peginterferon alfa‐2a (40kD) (Pegasys) in combination with ribavirin (RBV) versus interferon alfa‐2b plus RBV [EASL abstract]. Journal of Hepatology 2002;36(1):110.
Hinrichsen 2002 {published data only}
-
- Hinrichsen H, Buggisch P, Nasser S, Foelsch UR. A prospective randomised 24 week trial of peg‐interferon alpha2b plus ribavirin versus standard combination therapy in untreated hepatitis C genotype 2 and 3 patients [AASLD abstract]. Hepatology 2002;36(4 Pt 2):311A.
Horsmans 2008 {published data only}
-
- Horsmans Y, Colle I, Vlierberghe H, Langlet P, Adler M, Bourgeois N, et al. Weekly pegylated Interferon alpha‐2b vs daily interferon a‐2b versus standard regimen of Interferon a‐2b in the treatment of patients with chronic hepatitis C virus infection. Acto Gastro‐Enterologica Belgica 2008;71(3):293‐7. - PubMed
Izumi 2004 {published data only}
-
- Izumi N, Asahina Y, Kurosaki M, Uchihara M, Nishimura Y, Inoue K, et al. A comparison of the exponential decay slope between PEG‐IFN alfa‐2b/ribavirin and IFN alfa‐2b/ribavirin combination therapy in patients with chronic hepatitis C. Intervirology 2004;47:102‐7. - PubMed
Lee 2005 {published data only}
-
- Lee SD, Yu ML, Cheng PN, Lai MY, Chao YC, Hwang SJ, et al. Comparison of a 6‐month course peginterferon alpha‐2b plus ribavirin and interferon alpha‐2b plus ribavirin in treating Chinese patients with chronic hepatitis C in Taiwan. Journal of Viral Hepatitis 2005;12:283‐91. - PubMed
Mangia 2005 {published data only}
-
- Mangia A, Ricci GL, Persico M, Minerva N, Caretta V, Bacca D, et al. A randomized controlled trial of peginterferon alpha‐2a (40 kD) or interferon alpha‐2a plus ribavirin and amantadine vs interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C. Journal of Viral Hepatitis 2005;12(3):292‐9. - PubMed
Manns 2001 {published data only}
-
- Buti M, Medina M, Casado MA, Wong JB, Fosbrook L, Esteban R. A cost‐effectiveness analysis of peginterferon alfa‐2b plus ribavirin for the treatment of naive patients with chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2003;17:687‐94. - PubMed
-
- Condat B. Peginterferon alpha‐2b plus ribavirin compared with interferon alpha‐2b and ribavirin for the treatment of chronic hepatitis C: A randomized trial. Hepato‐Gastroenterology 2002;9(2):141‐2.
-
- Gish R, Bzowej N, Brooks L, Brass C, Weng W. Treatment with pegylated interferon alfa‐2b in combination with ribavirin improved health‐related quality of life compared with interferon alfa‐2b plus ribavirin in chronic hepatitis C [AASLD abstract]. Hepatology 2002;36(4 Pt 2):582A.
-
- Jen J, Laughlin M, Chung C, Heft S, Affrime MB, Gupta SK, et al. Ribavirin dosing in chronic hepatitis C: application of population pharmacokinetic‐pharmacodynamic models. Clinical Pharmacology and Therapeutics 2002;72(4):349‐61. - PubMed
-
- Manns MP, McHutchison JG, Gordon S, Rustgi V, Shiffman ML, Lee WM, et al. Peginterferon alfa‐2b plus ribavirin compared to interferon alfa‐2b plus ribavirin for the treatment of chronic hepatitis C: 24 week treatment analysis of a multicenter, multinational phase III randomized controlled trial [AASLD abstract]. Hepatology 2000;32(4 Pt 2):297A.
Napoli 2005 {published data only}
-
- Napoli N, Giannelli G, Parisi CV, Antonaci A, Maddalena G, Antonaci S. Predictive value of early virological response to treatment with different interferon‐based regimens plus ribavirin in patients with chronic hepatitis C. New Microbiologica 2005;28:13‐21. - PubMed
Nevens 2010 {published data only}
-
- Nevens F, Vlierberghe H, D'Heygere E, Delwaide J, Adler M, Henrion J, et al. A randomised, open‐label, multicenter study evaluating the efficacy of peginterferon alfa‐2a versus interferon alfa‐2a, in combination with ribavirin, in naïve and relapsed chronic hepatitis C patients. Acta Gastroenterologica Belgica 2010;73:223‐8. - PubMed
PRETTY 2005 {published data only}
-
- Mangia A, Cimino L, Persico M, Demelia L, Rumi M, Spinzi G, et al. Enhanced response to peginterferon‐alpha‐2a‐based triple therapy in previously non‐responsive chronic hepatitis C: final results of PRETTY study. Journal of Hepatology 2005;42(2):200‐1.
Rahman 2007a {published data only}
-
- Rahman F, Schuchmann M, Lohr HF, Link R, Buggisch P, Fuchs M, et al. Daily consensus interferon versus peg‐interferon alfa2b with weight based or 800 mg ribavirin in treatment‐naive patients with chronic hepatitis C genotype 1. Hepatology 2007;46(4 Suppl 1):826A.
Rahman 2007b {published data only}
-
- Rahman F, Schuchmann M, Lohr HF, Link R, Buggisch P, Fuchs M, et al. Daily consensus interferon versus peg‐interferon alfa2b with weight based or 800 mg ribavirin in treatment‐naive patients with chronic hepatitis C genotypes 2 or 3. Hepatology 2007;46(4 Suppl 1):826A.
Roffi 2008 {published data only}
-
- Roffi L, Colloredo G, Poggio P, Fornaciari G, Castagnetti E, Ceriani R, et al. Efficacy and safety of low doses of interferon (IFN) alfa‐2b plus ribavirin (RBV) in advanced HCV related liver disease [AASLD abstract]. Hepatology 2004;40(4 Suppl 1):322A.
-
- Roffi L, Colloredo G, Pioltelli P, Bellati G, Pozzpi M, Parravicini P, et al. Pegylated interferon‐alpha2b plus ribavirin: an efficacious and well‐tolerated treatment regimen for patients with hepatitis C virus related histologically proven cirrhosis. Antiviral Therapy 2008;13(5):663‐73. - PubMed
Scotto 2005 {published data only}
-
- Scotto G, Fazio V, Palumbo E, Cibelli DC, Saracino A, Angarano G. Treatment of genotype 1b HCV‐related chronic hepatitis: efficacy and toxicity of three different interferon alfa‐2b/ribavirin combined regimens in naive patients. New Microbiologica 2005;28:23‐9. - PubMed
Shobokshi 2003 {published data only}
-
- Shobokshi A, Serebour FE, Skakni L, Al‐Jasser N, Tantawi AO, Sabah A, et al. Combination therapy of peginterferon alfa‐2a (40kD) (Pegasys(R)) and ribavirin (Copegus(R)) significantly enhance sustained virological and biochemical response rate in chronic hepatitis C genotype 4 patients in Saudi Arabia [AASLD abstract]. Hepatology 2003;38(4 Suppl 1):636A.
-
- Shobokshi O, Serebour F, Skakni L, Tantawi A, Dinish T, Al Quaiz M, et al. Efficacy of pegylated (40kDa) IFN alfa‐2a (Pegasys) plus ribavirin in the treatment of hepatitis C genotype 4 chronic active patients in Saudi Arabia. Journal of Hepatology 2002;36(Suppl 1):129.
-
- Shobokshi O, Serebour FE, Skakni L, Al‐Jaser N, Tantawe AO, Sabah A, et al. Early virological response at week 12 has positive predictive value for end of treatment response for hepatitis C virus genotype 4 chronic active hepatitis cases treated with combination therapy of pegylated interferon plus ribavirin. Saudi Medical Journal 2003;24(Suppl 2):S92‐S93.
-
- Shobokshi O, Serebour FE, Skakni L, Al‐Jaser N, Tantawe AO, Sabah A, et al. Pegylated interferon alfa‐2a (40KDA) as a monotherapy or in combination with ribavirin significantly improves end of treatment response rate in hepatitis C virus genotype 4 chronic active hepatitis patients. Saudi Medical Journal 2003;24(Suppl 2):S92‐S93.
Sjögren 2007 {published data only}
-
- Sjogren MH, Sjogren R, Lyons MF, Ryan M, Santoro J, Smith C, et al. Antiviral response of HCV genotype 1 to consensus interferon and ribavirin versus pegylated interferon and ribavirin. Digestive Diseases and Sciences 2007;52(6):1540‐7. - PubMed
Thakeb 2003 {published data only}
-
- Thakeb FIA, Omar MM, Awady MM, Isshak SY. Randomized controlled trial of peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus‐genotype 4 among Egyptian patients [AASLD abstract]. Hepatology 2003;38(4 Suppl 1):278A.
Tsubota 2005 {published data only}
-
- Tsubota A, Arase Y, Someya T, Suzuki Y, Suzuki F, Saitoh S, et al. Early viral kinetics and treatment outcome in combination of high‐dose interferon induction vs. pegylated interferon plus ribavirin for naive patients infected with hepatitis C virus of genotype 1b and high viral load. Journal of Medical Virology 2005;75:27‐34. - PubMed
Wakil 2006 {published data only}
-
- Wakil RM, Montasser MF, Mansour MA, Salman TA, El‐Batanony MH, Helail EH, et al. Peginterferon alpha‐2b plus ribavirin versus interferon alpha‐2b plus ribavirin for treatment of chronic hepatitis C in treatment‐naive Egyptian patients. Journal of Clinical Virology 2006;36:S142‐S143.
References to studies excluded from this review
ACTG 2005 {published data only}
Ali 2010 {published data only}
-
- Ali S, Nazir G, Khan SA, Iram S, Fatima F. Comparative therapeutic response to pegylated interferon plus ribavirin versus interferon alpha‐2b in chronic hepatitis C patients. Journal of Ayub Medical College 2010;22:127‐30. - PubMed
APRICOT 2004 {published data only}
-
- Mauss S, Valenti W, DePamphilis J, Duff F, Cupelli L, Passe S, et al. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon‐based therapy. AIDS 2004;18(13):F21‐F25. - PubMed
-
- Torriani FJ, Ribeiro RM, Gilbert TL, Schrenk UM, Clauson M, Pacheco DM, et al. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. Journal of Infectious Diseases 2003;188:1498‐507. - PubMed
-
- Torriani FJ, Rodriguez‐Torres M, Rockstroh JK, Lissen E, Gonzalez‐Garcia J, Lazzarin A, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection in HIV‐infected patients. New England Journal of Medicine 2004;351(5):438‐50. - PubMed
Asahina 2004 {published data only}
-
- Asahina Y, Izumi N, Onuki Y, Ueda K, Nishimura Y, Nakanishi H, et al. Enhanced interferon (IFN)‐stimulated gene expression and HCV dynamics in patients treated with pegylated IFN alfa 2b and ribavirin: effect of pharmacokinetic change of IFN and ribavirin. Hepatology 2004;40(4 Suppl 1):338A.
Gromova 2004 {published data only}
-
- Gromova NI, Bogomolov BP. Comparative efficacy of combined treatment with Intron a and Pegintron in combination with ribavirin in patients with chronic hepatitis C. Terapevticheskii Arkhiv 2004;76(2):31‐5. - PubMed
Laguno 2004 {published data only}
-
- Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez‐Tapias JM, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for treatment of HIV/HCV co‐infected patients. AIDS 2004;18(13):F27‐F36. - PubMed
RIBAVIC 2004 {published data only}
-
- Carrat F, Bani‐Sadr F, Pol S, Rosenthal E, Lunel‐Fabiani F, Benzekri A, et al. Pegylated interferon alfa‐2b vs standard interferon alfa‐2b, plus ribavirin, for chronic hepatitis C in HIV‐infected patients. JAMA 2004;292(23):2839‐48. - PubMed
-
- Lunel F, Pivert A, Payan C. Comparison of HCV RNA and HCV core antigen kinetics in the follow up of a therapeutic protocol in HCV‐HIV co‐infected patients (RIBAVIC). Journal of Hepatology 2004;40(Suppl 1):144‐5.
-
- Pol S, Carrat F, Bani‐Sadr F, Rosenthal E, Lunel F, Morand P, et al. Final results of ANRS HC02‐RIBAVIC: a randomized controlled trial of pegylated‐interferon alfa‐2b plus ribavirin vs interferon alfa‐2b plus ribavirin for the initial treatment of chronic hepatitis C in HIV co‐infected [AASLD abstract]. Hepatology 2004;40(4):315A.
-
- Pol S, Carrat F, Bani‐Sadr F, Rosenthal E, Lunel F, Morand P, et al. Final results of ANRS HC02‐RIBAVIC: a randomized controlled trial of pegylated‐interferon alpha‐2b plus ribavirin vs interferon alpha‐2b plus ribavirin for naive HCV‐HIV co‐infected patients. Journal of Hepatology 2005;42(Suppl 2):10‐1.
Additional references
Adeel 2009
-
- Adeel AB, Xiaoqiang Wang, Charity GM. Effect of hepatitis C virus and its treatment on survival. Hepatology 2009;50(2):387‐92. - PubMed
Aljumah 2013
Awad 2010
Bailon 2001
-
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung W‐J, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis. Bioconjugate Chemistry 2001;12(2):195‐202. - PubMed
Bangalore 2008
-
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli F. Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet 2008;372(9654):1962‐76. - PubMed
Benvegnu 2001
-
- Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma development in hepatitis B virus and hepatitis C virus‐related cirrhosis. Antiviral Research 2001;52:199‐207. - PubMed
Bradburn 2006
-
- Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2006;26(1):53‐77. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Apparently conclusive meta‐analyses may be inconclusive—trial sequential analysis adjustment for random error risk in conclusive Cochrane neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Brok 2009a
Chan 2013
Chander 2002
-
- Chander G, Sulkowski MS, Jenckes MW, Torbenson MS, Herlong HF, Bass EB, et al. Treatment of chronic hepatitis C: a systematic review. Hepatology 2002;36(S1):135‐44. - PubMed
Constant 2005
-
- Constant A, Castera L, Dantzer R, Couzigou P, Ledinghen V, Demotes‐Mainard J, et al. Mood alterations during interferon‐alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. Journal of Clinical Psychiatry 2005;66:105‐23. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA—Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 28 May 2013).
Davis 1999
-
- Davis GL. Hepatitis C virus genotypes and quasispecies. American Journal of Medicine 1999;107(6B):S21‐S26. - PubMed
DeMets 1987
-
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dhillon 2010
EASL 2012
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of Hepatology 2011;55:245‐64. - PubMed
Fattovich 2002
-
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, European Concerted Action on Viral Hepatitis (EUROHEP). Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. American Journal of Gastroenterology 2002;97(11):288‐95. - PubMed
Foster 2004
-
- Foster GR. Pegylated interferons: chemical and clinical differences. Alimentary Pharmacology & Therapeutics 2004;20(8):825‐30. - PubMed
Ghany 2009
Glue 2000
-
- Glue P, Fang JWS, Rouzier‐Panis R, Raffanel C, Sabo R, Gupta SK, et al. Pegylated interferon‐[alpha]2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clinical Pharmacology and Therapeutics 2000;68(5):556‐67. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 12. Art. No.: LIVER.
Gurusamy 2013
-
- Gurusamy KS, Wilson E, Koretz RL, Allen VB, Davidson BR, Burroughs AK, et al. Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?. PLoS ONE 2013;8(12):e83313. - PMC - PubMed
Guyatt 2008
Hadziyannis 2004
-
- Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon alfa‐2a (40 kilodaltons) and ribavirin combination therapy in chronic hepatitis C: randomized study of the effect of treatment duration and ribavirin dose. Annals of Internal Medicine 2004;140:346–55. - PubMed
Hauser 2014
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hirofumi 2009
Hodgson 2003
-
- Hodgson HJF. Viral hepatitis—clinical aspects. In: Warrell DA, Cox TM, Firth JD, Benz EJ Jr editor(s). Oxford Textbook of Medicine. 4th Edition. Oxford/ New York: Oxford University Press, 2003.
Hollis 1999
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Khuroo 2004
-
- Khuroo MS, Khuroo MS, Dahab ST. Meta‐analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Alimentary Pharmacology and Therapeutics 2004;20(9):931‐8. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koretz 2013
Krleza‐Jeric 2005
Kumar 2002
-
- Kumar KS, Russo MW, Borczuk AC, Brown M, Esposito SP, Lobritto SJ, et al. Significant pulmonary toxicity associated with interferon and ribavirin therapy for hepatitis C. American Journal of Gastroenterology 2002;97:2432‐40. - PubMed
Lim 2010
-
- Lim JW, Shin MC. Pegylated‐interferon‐associated retinopathy in chronic hepatitis patients. Ophtalmologica 2010;224:224‐9. - PubMed
Lundh 2012
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Moher 2012
-
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10:28‐55. - PubMed
Myers 2002
OPTN 2008
-
- United Network for Organ Sharing. Chapter IV; Liver and Intestine Transplantation in the United States, 1998‐2007. optn.transplant.hrsa.gov/ar2008/chapter_iv_AR_cd.htm?cp=5 (accessed 27 May 2013).
Penin 2004
Raison 2005
Reddy 2001
-
- Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40‐kd) interferonalpha‐2a compared with interferon alpha‐2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001;33(2):433–8. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roomer 2010
-
- Roomer R, Hansen BE, Janssen HL, Knegt RJ. Thrombocytopenia and the risk of bleeding during treatment with peginterferon alfa and ribavirin for chronic hepatitis C. Journal of Hepatology 2010;53:455‐9. - PubMed
Rosenberg 2001
-
- Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. Journal of Molecular Biology 2001;313:451‐64. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. - PubMed
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Seeff 2002
Seeff 2009
Shepherd 2005
-
- Shepherd J, Brodin H, Kan FT, Cave CB, Waugh NR, Price A, et al. Clinical‐ and cost‐effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. International Journal of Technology Assessment in Health Care 2005;21(1):47‐54. - PubMed
Siebert 2005
-
- Siebert U, Sroczynski G, German Hepatitis C Model GEHMO Group, HTA Expert Panel on Hepatitis C. Effectiveness and cost‐effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. International Journal of Technology Assessment in Health Care 2005;21(1):55‐65. - PubMed
Slavenburg 2010
Soza 2002
-
- Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology 2002;36:1273‐9. - PubMed
Sulkowski 2004
-
- Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha‐2b plus ribavirin combination therapy for chronic hepatitis C virus infection. Journal of Viral Hepatitis 2004;11:243‐50. - PubMed
Sulkowski 2011
-
- Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck‐Radosavljevic M, et al. Management of adverse effects of Peg‐IFN and ribavirin therapy for hepatitis C. Nature Reviews. Gastroenterology & Hepatology 2011;8:212‐23. - PubMed
Thorlund 2009
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 April 2013).
van Regenmortel 2000
-
- Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, et al. Virus Taxonomy: The Classification and Nomenclature of Viruses. The Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 2000:599‐621.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
WHO 1999
-
- World Health Organization (WHO). Global surveillance and control of hepatitis C. Journal of Viral Hepatitis 1999;6(1):35‐47. - PubMed
WHO 2009
-
- WHO. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed 27 May 2013).
Wood 2008
Zaman 2003
-
- Zaman A, Fennerty MB, Keeffe EB. Peginterferon vs. standard interferon in the treatment of chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2003;18(7):661‐70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

