The state of norovirus vaccines
- PMID: 24585561
- PMCID: PMC4036685
- DOI: 10.1093/cid/ciu120
The state of norovirus vaccines
Abstract
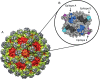
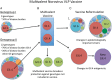
Noroviruses represent the most important cause of acute gastroenteritis worldwide; however, currently no licensed vaccine exists. Widespread vaccination that minimizes overall norovirus disease burden would benefit the entire population, but targeted vaccination of specific populations such as healthcare workers may further mitigate the risk of severe disease and death in vulnerable populations. While a few obstacles hinder the rapid development of efficacious vaccines, human trials for virus-like particle (VLP)-based vaccines show promise in both immune response and protection studies, with availability of vaccines being targeted over the next 5-10 years. Ongoing work including identification of important norovirus capsid antigenic sites, development of improved model systems, and continued studies in humans will allow improvement of future vaccines. In the meantime, a better understanding of norovirus disease course and transmission patterns can aid healthcare workers as they take steps to protect high-risk populations such as the elderly and immunocompromised individuals from chronic and severe disease.
Keywords: norovirus; vaccine.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect. 2006;12:69–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

